Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.2 Lisboa jun. 2016
ORIGINAL ARTICLE
Increased peritoneal dialysis utilization and improved patient survival over a 20-year period: data from a Portuguese Peritoneal Dialysis Unit
Brigite Aguiar, Luís Rodrigues, Andreia Borges, Helena Sá, Rui Alves, Mário Campos
Nephrology Department, Centro Hospitalar e Universitário de Coimbra‑Hospitais
Universitários de Coimbra (CHUC‑HUC), Portugal.
ABSTRACT
Background: Peritoneal dialysis (PD) is an established renal replacement therapy, mainly performed at home. There is a general perception that the use of PD is declining worldwide. As countries look to develop dialysis programmes to manage the growing burden of end‑stage renal disease (ESRD), it is important to place patterns of PD use in the global context. Although there has been an improvement in PD patient and technique survival over the last years, this modality still remains underutilized in Portugal. Objectives: The primary aim was to evaluate patient and technique survival in a single centre in Portugal over a 20‑year period, comparing the last decade with the prior decade, and to identify clinically important factors that predict patient mortality and technique failure. The secondary aim was to determine the main reasons for patient dropout from PD. Methods: Historical cohort study including patients initiating PD between January 1992 and December 2012. Multivariate Cox regression models were developed using baseline candidate variables to predict all‑cause mortality and technique survival. Results: A total of 184 patients were included (59.2% male, mean age 48.7 ± 16.9 years), on PD for 24.7 ± 21.2 months. There was an increase in PD use between the first and last decades (79 vs. 105 patients), especially in automated PD (48.1% vs. 60.0%). The main causes of PD drop out were death (34.2%), renal transplant (29.3%) and switch to HD (18.5%) due to inadequate ultrafiltration (38.2%), and peritonitis and access‑related infections (29.4%). Patient survival at 5 years was 51.9% in the first decade, and 78.1% in the last decade (p < 0.001). The PD technique survival did not change from the first to the last decade. The presence of prior haemodialysis and diabetes mellitus were predictors of mortality. Conclusion: Over the last two decades, there has been an increase in PD use, and an improvement in patient survival in our Unit.
Key‑Words: Patient survival; PD dropout; peritoneal dialysis; technique failure.
INTRODUCTION
The incidence and prevalence of end‑stage renal disease (ESRD) in Portugal are among the highest in the world. According to data from the Registry of the
Portuguese Society of Nephrology, in 2012, the incidence of ESRD was 219 per million population (pmp), of which 197.3 pmp on haemodialysis (HD) and 20.8 pmp on peritoneal dialysis (PD). At the end of the same year, there were 17.641 patients on renal replacement therapy (RRT), of which only 742 patients were on PD.
However, the use of this modality in Portugal has been increasing: in 1997, patients undergoing PD represented 5.6% of the patients initiating dialysis in that year, while in 2012 this figure has increased to 9.5%1. Peritoneal dialysis has been available in Portugal since the early 1980s, but our PD programme at the University Hospital of Coimbra started in 1988.
Some people propose the use of PD as the initial treatment modality in patients with ESRD2, emphasizing the potential advantages of PD over HD3,4, which include being performed mainly at home, at a lower cost5, with better health‑related quality of life6, and with better preservation of residual renal function (RRF)7.
Over the years, patients undergoing PD have shown an improvement in clinical outcomes, associated with technical progress in connecting systems, the use of more biocompatible solutions, the adhesion to clinical practice guidelines, and physicians accumulated experience.
However, several risk factors are independently associated with poor survival in the population of patients undergoing PD. These include older age, male gender, low socioeconomic status, and the presence of comorbid conditions such as diabetes mellitus, cardiovascular disease, and malnutrition8‑10.
For each 1‑year increase in patient age, the risk of death increases by 4%, and patients with diabetes have a 30% increase in the risk of death as compared with non‑diabetic patients11. Some studies suggest that ESRD patients in whom PD is the initial treatment modality, have a 28% lower risk of death as compared with patients undergoing PD after switching from HD7. Other studies have identified other risk factors associated with technique failure, such as low RRF, increased peritoneal transport status, type of PD (continuous ambulatory peritoneal dialysis – CAPD, or assisted peritoneal dialysis – APD), and centre size12. In the end, the success of a PD programme is highly dependent upon other important factors, such as peritonitis rate and catheter‑related infections.
OBJECTIVES
The primary aim of the present study was to assess longitudinal trends in the use of PD, the PD technique and patient survival over a 20‑year period in a single centre in Portugal, comparing survival in the last decade with that in the prior decade, and to identify predictors of patient and PD technique survival. The secondary aim was to determine the main reasons for patient dropout from PD treatment, comparing two distinct decades (1992‑2001 and 2002‑2012).
SUBJECTS AND METHODS
This historical cohort study included all patients that initiated PD in our Unit, from January 1992 to December 2012. We evaluated 192 and excluded 8 patients for the following reasons: age under 18 years and a follow‑up of less than one month. A total of 184 patients were included in the final cohort and, in order to analyse the trends in patient characteristics over time, this cohort was divided into two separate periods: 1992‑2001 and 2002‑2012.
For each period, clinical data regarding demographic variables (gender, age at start of PD, aetiology of ESRD), the Charlson comorbidity index (CI), serum albumin, presence or absence of diabetes mellitus, type of RRT prior to PD (kidney transplant, HD, dialysis‑naive), PD‑related variables (PD modality – continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis (APD), duration of PD, use of icodextrine, and peritonitis event rate. Baseline peritoneal transport was not available for the analysis, since the peritoneal equilibration test was not routinely performed until 2002.
The PD dropout causes included switch to HD, renal transplantation and death. Switch to HD was divided into broad categories according to the underlying reason: infection (peritonitis/exit site infections and other), inadequate dialysis (ultrafiltration failure/low dialysis dose), catheter‑related problems, and psychological causes (patient decision, insufficient patient support).
Causes of death were divided into five categories: cardiovascular disease, peritonitis‑related, infections other than peritonitis, malignancy and unknown causes. Death within 30 days after switch to HD was also ascertained.
Peritonitis was defined according to the International Society of Peritoneal Dialysis (ISPD) guidelines by the presence of two of the following criteria: 1) abdominal pain; 2) dialysate leukocyte count > 100 cells/μL, with at least 50% neutrophils; and 3) positive dialysate microbiological culture13. All peritonitis episodes occurring between the 1st January 1992 and 31st December 2012 were included. Follow‑up was censored at the time of renal transplantation, switch to HD, or 31st of December 2012, whichever came first.
PD Unit
The Y‑set systems were implemented in our Unit since 1989. Straight double‑cuffed Tenckhoff catheters Tenckhoff catheters were used, placed after antibiotic prophylaxis via a minilaparotomy performed by an experienced team of surgeons.
Since 2002, all patients have used a dialysate with low glucose degradation products (GDP), with neutral pH, and icodextrin was available to patients with ultrafiltration failure.
Automated peritoneal dialysis was prescribed for the first time in 1997. It was initially prescribed only for anuric patients, but now, due to patient logistic/ professional reasons, it is the most prescribed modality.
Peritoneal equilibration test (PET) was introduced in our Unit in 2002 and, since then, it has been possible to determine solute transport rate by measuring the creatinine saturation of the PD fluid (dialysate/plasma ratio for creatinine or D/P creatinine) at four hours.
Peritoneal dialysis adequacy is usually quantified in terms of weekly urea Kt/V, and in our Unit it was performed routinely twice a year. We found a weekly Kt/V of 2.4 ± 0.8 in our study, which is higher than the value recommended by current guidelines14.
Statistical analysis
Continuous symmetrically distributed data is expressed as mean ± standard deviation (SD). Asymmetrically distributed data is expressed as median and range. Categorical data are expressed as absolute numbers and percentages. Chi‑square test was used for categorical variables. Student´s t‑test was used for analysing clinical parameters.
Patient and technique survival were analysed by Kaplan‑Meier method and log‑rank test was used to compare time to event between groups. In the technique survival analysis, the switch to HD was considered the final event. The PD patients who received a kidney transplant, were transferred to another centre or showed recovery of renal function were censored and considered to be lost to follow‑up.
In patient survival analysis, death was the event. Patients who received a kidney transplant, were switched to HD, showed recovery of renal function, or were transferred to another centre, were censored and considered to be lost to follow‑up.
Cox regression analysis was performed for patient and technique survival in order to take into account the relative effects of various risk factors. In the current analysis, demographic and PD‑related variables were the risk factors taken into account. Peritonitis rate was expressed as episodes/patient.year. A p‑value less than 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS software version 21 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Patient characteristics
The mean age at the beginning of PD was 49 ± 17 years, with male predominance (59.2%), 19% were over the age of 65 years, 21.2% were diabetic, 50.0% were previously treated with HD or renal transplantation and 25% of the patients were anuric at the start of PD. The main reasons for starting PD were chronic kidney disease (CKD) progression in 50.0%, switch from HD in 41.8% and loss of a functioning renal allograft in 8.2%. Patient preference occurred in 58.7%, and significantly increased between the first and the second decades of our programme (35.2% vs. 64.8%, p = 0.001). Vascular access failure while on HD was the reason for switch to PD in 41.3%. Mean follow‑up was 24.7 ± 21.2 months. Automated peritoneal dialysis was the predominant technique (54.9%), with a shorter follow‑up (23.5 ± 19.3 vs. 26.1 ± 12.3 months in CAPD).
Differences between decades
There was an increase in PD use between the two time periods. Comparison of patient characteristics between the first and last decades is presented in Table I. No statistically significant differences were observed between the two decades, as far as age, gender, CI, follow‑up time or cause of CKD are concerned.
In the first decade, a higher proportion of PD patients had switched from HD (p = 0.017), and had diabetes mellitus (p = 0.023), as compared with the last decade.
Vascular access failure while on HD, as a reason for switching to PD, decreased significantly between the first and last decades of the follow‑up period (51.9% vs. 33.3%, p < 0.001). As anticipated, a higher use of PD as the first RRT and of APD was observed in the last decade.
Reasons for peritoneal dialysis dropout
The reasons for PD dropout are depicted in Table II. In this cohort, the two main reasons for the switch to HD were inadequate ultrafiltration (38.2%), and peritonitis and access‑related infections (29.4%) (Table III).
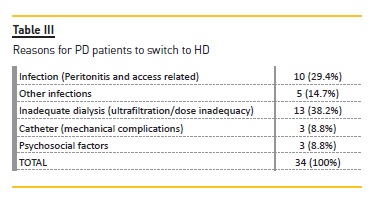
Of the 10 patients who switched to HD due to peritonitis, eight patients showed a gram‑negative bacteria (Escherichia coli (two), Klebsiella spp (two), Pseudomonas aeruginosa (two), Enterococcus faecalis, Corynebacterium spp) and two showed a gram‑positive bacteria (both with Staphylococcus aureus). Five PD patients (14.7%) switched to HD due to other infectious complications, three (8.8%) due to catheter dysfunction and three (8.8%) due to patient choice.
Peritonitis occurred in 72 patients (39.1%), with a total of 91 episodes (1.2 episodes/patient). The overall peritonitis rate in the studied period was 0.31 episodes/patient.year.
Over the follow‑up period, of the 76 PD patients who switched from HD due to vascular access failure, 50 (65.8%) died, 10 (13.2%) received a kidney transplant, and seven (9.2%) returned to HD. These patients were significantly older (mean age 58.4 ± 14.9 years) than those who chose PD as the first RRT (mean age 41.8 ± 14.7 years, p = 0.001), possibly partially explaining the difference in outcomes observed between the two time periods. Of the 108 patients who chose PD as the first RRT, only 13 (12.0%) died, 27 (25.0%) returned to HD, and 44 (40.7%) received a kidney transplant during the follow‑up period (Table IV).
Patient survival analysis
During the observation period, 63 patients (34.2%) died while on PD. The leading cause of death was cardiovascular disease, occurring in 29 patients (46.0%). Infections not related to the PD technique were the cause of death in 11 patients (17.5%) and peritonitis in four patients (4.5%). Gram‑negative bacteria (Escherichia coli, Pseudomonas aeruginosa) were responsible for peritonitis in two patients, a gram‑positive (Staphylococcus aureus MR) microorganism in one patient and a fungus (Candida albicans) in another. Four patients died due to malignancy
and the cause of death was unknown in 14 patients.Patient survival was 86.4%, 72.8% and 66.8% at 1, 3 and 5 years, respectively. A significant decrease in mortality was observed in the last decade as compared with the first (Table V).
Several factors that may have influenced patient survival were examined. Gender and race were not associated with greater mortality risk. Using univariate Cox regression analysis, diabetes mellitus, older age, and prior HD were associated with lower survival. Using multivariate Cox regression analysis, the presence of prior HD (HR 4.7, 95% CI: 3.6‑6.7, p < 0.02) and diabetes mellitus (HR 2.4, 95% CI: 2.1 – 5.4, p < 0.02) were predictors of mortality (Figs. 1 and 2).
Technique survival analysis
Technique survival was 85.9%, 64.7% and 56.5% at 1, 3 and 5 years, respectively. Median survival time was 39 months. Technique survival was not affected by whether patients were undergoing PD after switching from HD (due to access failure) or as the first RRT. Using a Cox proportional hazard model, none of the variables examined were significantly associated with risk of technique failure. No statistically significant difference was observed between the first and last decades regarding technique survival (Table V).
DISCUSSION
The present study evaluated patient characteristics, physicians practice patterns, and patient and technique outcomes, in a historical cohort of PD patients, over a two‑decade period, comparing the last decade (2002‑2012) with the earlier decade (1992‑2001).
The characteristics of the population in the present study are similar to those of patients undergoing dialysis in Portugal. Diabetic nephropathy was an important cause of chronic kidney disease (CKD) in our cohort, as would be anticipated, since diabetes mellitus is a major cause of CKD in Portugal, according o data from the Portuguese Society of Nephrology Registry1. Data from the 2012 Annual Report of that Registry1, showed that the PD population in Portugal is younger (mean age: 52 years) than the overall ESRD population (mean age: 69 years). This study showed similar data.
The outcomes of PD usually assessed are patient and technique survival and peritonitis incidence.
There are now several large cohort studies from different countries reporting these outcomes of patients on PD15. It should be noted that, globally, the overall patient survival rate in this study was 66.8% at 5 years, and patients showed an improved survival within more recent years (2002‑2012).
Lai and Lo, in a study from Hong Kong, reported a 5‑year survival of 57%16. In 2006, the United States Renal Data System (USRDS) reported a 5‑year survival of 32% in the USA17. Five‑year survival in France and Denmark was 27% and 40%, respectively18,19. The difference in survival between our cohort and these other cohorts is possibly partially explained by differences in age: our patients had a median age of 49 years, while the Danish and French cohorts had mean ages of 60 and 53 years, respectively18,19. These differences in patient survival are possibly also influenced by other factors, such as differences in race, genetic background, presence of diabetes mellitus, malnutrition, number of comorbid diseases, low RRF, prior HD and dialysis centre related issues.
Dialysis patients have a high prevalence of traditional cardiovascular risk factors. However, in dialysis patients, the therapies addressing those factors have not been effective in reducing cardiovascular events or mortality.
Addressing modifiable non‑traditional risk factors, unique to the CKD population, might improve mortality rates, but this remains unproven. Current evidence suggests that we should probably focus on the preservation of RRF, peritoneal membrane integrity, and prevention of PD‑related infections, which are factors strongly associated with long‑term survival in PD patients9.
In this study, the presence of diabetes mellitus and prior HD were found to be predictors of mortality. Several other studies have also shown that diabetes mellitus and low plasma albumin are repeatedly linked to poor clinical outcomes20,21, and predict worsening cardiovascular morbidity22. Some studies also reported that women have worse survival on PD23,24, however, we could not confirm this finding.
It has been suggested that centre size is inversely correlated with mortality25, and this could be an additional explanation for the lower mortality observed in this study on the last decade. This centre effect could be linked to several factors, including better infection control following adherence to clinical practice guidelines since 2002, better catheter access practices due to experience acquired by our surgical team, increasing experience of our physicians and increased nurse‑to‑patient ratio.
However, it is also possible that the improved survival in the last decade could be the result of a healthier group of patients initiating PD, since we observed an increase in the proportion of PD patients who chose PD as the first RRT, and a lower number of PD patients switching from HD due to vascular access failure.
Technique survival also varies widely among centres. Our technique survival at five years was 56.5%, which was a poor survival as compared with that of studies from Spain and Japan (64.2% and 70%, respectively)26.
In our study, we did not find any impact of PD modality (APD/CAPD) on patient and technique survival. Furthermore, age and the presence of diabetes mellitus had no effect on technique survival, suggesting that PD can be successful in these groups.
Peritonitis remains a major cause for PD patients switching to HD, especially within the first two years of initiating PD. Repeated episodes of peritonitis may lead to membrane failure with subsequent technique failure, substantial morbidity and mortality9. Prevention of exit‑site infections and appropriate treatment of peritonitis with established protocols is critical for a successful PD programme. Several studies have found a lower peritonitis rate in APD than CAPD, likely related to fewer connections and disconnections, however, this data was not confirmed by others27,28. This association could not be assessed in this study due to the small sample size.
This study has several limitations. In addition to its retrospective design, it is a single‑centre study with a small sample size and a high risk of bias, precluding the establishment of definitive conclusions. Despite these limitations, reports regarding the long‑term outcome of PD patients are important tools for the continuous development of this therapy.
CONCLUSION
The present study presents data on the long‑term outcomes of a cohort of PD patients in a single Portuguese PD Unit, and suggested an increase in patient survival in the last decade as compared with the prior decade. This improved survival was associated with an increased PD use. These results need to be confirmed in a larger cohort.
References
1. Portuguese Registry of Renal Replacement Therapy, 2012 Annual Data Report. Available at http://www.spnefro.pt/comissoes_Gabinete_registo_2012/registo_2012. [ Links ]
2. Blake PG. Integrated end‑stage renal disease care: the role of peritoneal dialysis. Nephrol Dial Transplant 2001;16(Suppl 5):61‑66. [ Links ]
3. Schaubel DE, Morrison HI, Fenton SS. Comparing mortality rates on CAPD/CCPD and hemodialysis. The Canadian experience: fact or fiction? Perit Dial Int 1998;18(5):478‑484. [ Links ]
4. U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End‑Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2009. [ Links ]
5. Shih YC, Guo A, Just PM, Mujais S. Impact of initial dialysis modality and modality switches on Medicare expenditures of end‑stage renal disease patients. Kidney Int 2005; 68(1): 319‑329. [ Links ]
6. Gokal R, Figueras M, Ollé A, Rovira J, Badia X. Outcomes in peritoneal dialysis and haemodialysis – a comparative assessment of survival and quality of life. Nephrol Dial Transplant 1999; 14(Suppl 6):24‑30. [ Links ]
7. Berlanga JR, Marrón B, Reyero A, Caramelo C, Ortiz A. Peritoneal dialysis retardation of progression of advanced renal failure. Perit Dial Int 2002; 22(2): 239‑242. [ Links ]
8. Unsal A, Koc Y, Basturk T, et al. Clinical outcomes and mortality in peritoneal dialysis patients: a 10‑year retrospective analysis in a single center. Clin Nephrol 2013; 80(4): 270‑279. [ Links ]
9. Pulliam J, Li NC, Maddux F, Hakim R, Finkelstein FO, Lacson E Jr. First‑year Outcomes of incident peritoneal dialysis patients in the United States. Am J Kidney Dis 2014; 64 (5):761‑769. [ Links ]
10. Joshi U, Guo Q, Yi C, et al. Clinical outcomes in elderly patients on chronic peritoneal dialysis: a retrospective study from a single center in China. Perit Dial Int 2014; 34 (3):299‑307. [ Links ]
11. Kendrick J, Teitelbaum I. Strategies for improving long‑term survival in peritoneal dialysis patients. Clin J Am Soc Nephrol 2010; 5(6):1123‑1131. [ Links ]
12. Guo A, Mujais S. Patient and technique survival on peritoneal dialysis in the United States: Evaluation in large incident cohorts. Kidney Int 2003; (88):3‑12. [ Links ]
13. Li PK, Szeto CC, Piraino B, et al.; International Society for Peritoneal Dialysis. Peritoneal dialysis‑related infections recommendations: 2010 Update. Perit Dial Int 2010;30(4):393–423. [ Links ]
14. Peritoneal Dialysis Adequacy 2006 Work Group. Clinical practice guidelines for peritoneal adequacy, update, 2006. Am J Kidney Dis 2006;48:S91‑S97 [ Links ]
15. Davies SJ, Philips L, Griffiths AM, Russell LH, F. Naish PF, Russell GI. What really happens to people on long‑term peritoneal dialysis. Kidney Int 1998; 54(6):2207‑2217. [ Links ]
16. Lai KN, Lo WK. Optimal peritoneal dialysis for patients from Hong Kong. Perit Dial Int 1999; 19(Suppl 3): 26‑31. [ Links ]
17. Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis 2007; 49 (Suppl 1):A6‑7, S1‑296. [ Links ]
18. Heaf JG, Lokkegaard H, Madsen M. Inicial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 2002; 17(1):112‑117. [ Links ]
19. Verger C, Ryckelynck JP, Duman M, et al. French peritoneal dialysis registry (RDPLF): Outline and main results. Kidney Int Suppl 2006; (103):S12‑20. [ Links ]
20. Sipahioglu MH, Aybal A, Unal A, Tokgoz B, Oymak O, Utas C. Patient and technique survival and factors affecting mortality on peritoneal dialysis in TurKey: 12 years experience in a single center. Perit Dial Int 2008; 28(3):238‑245. [ Links ]
21. Shiao CC, Kao TW, Hung KY, et al. Seven‑year follow up of peritoneal dialysis patients in Taiwan. Perit Dial Int 2009; 29(4):450‑457. [ Links ]
22. Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and mortality in end‑stage renal disease. J Am Soc Nephrol 1996; 7(5):728‑736. [ Links ]
23. Collins AJ, Hao W, Xia H, et al. Mortality risks of peritoneal dialysis and hemodialysis. Am J Kidney Dis 1999; 34(6):1065‑1074. [ Links ]
24. Vonesh EF, Moran J. Mortality in end stage renal disease: a reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 1999; 10(2):354‑365. [ Links ]
25. Schaubel DE, Blake PG, Fenton SS. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 2001; 60(4): 1517‑1524. [ Links ]
26. Nakamoto H, Kawaguchi Y, Suzuki H. Is technique survival on peritoneal dialysis better in Japan? Perit Dial Int 2006; 26 (2):136‑143. [ Links ]
27. Rodriguez‑Carmona A, Pérez Fontán M, Garcia Falcon T, Fernandez Rivera C, Valdés F. A comparative analysis on the incidence of peritonitis and exit‑site infection in CAPD and automated peritoneal dialysis. Perit Dial Int 1999; 19(3):253‑258. [ Links ]
28. Oo TN, Roberts TL, Collins AJ. A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am J Kidney Dis 2005; 45 (2):372‑380. [ Links ]
Brigite Aguiar
Nephrology Department
Centro Hospitalar e Universitário de Coimbra
Praceta Professor Mota Pinto 3000‑075
Coimbra, Portugal.
E‑mail: brigitebessacardoso@gmail.com
Conflict of interests statement: None to declare.
Received for publication: Dec 1, 2016
Accepted in revised form: Jan 1, 2016














