Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.2 Lisboa jun. 2016
CASE REPORT
Plasma exchange in hypertriglyceridaemic acute pancreatitis: case report
Cátia Cunha1, Ana Luísa Barbosa2, Susana Pereira1, Lilite Barbosa3, João Valente2, João Fernandes1
1 Department of Nephrology
2 Intermediate Care Unit
3 Department of General Surgery Centro Hospitalar de Vila‑Nova de Gaia/ Espinho, Portugal.
ABSTRACT
Severe hypertriglyceridaemia, defined as above 1000mg/dl, is the third most common cause of acute pancreatitis, and a potentially life‑threatening condition. The prognosis depends greatly on our ability to rapidly reduce serum triglycerides concentration. We report a case of a 41‑year‑old male admitted to our Emergency Department with symptoms of nausea, vomiting and abdominal pain in the right upper quadrant, whose workup revealed the presence of an extremely severe hypertriglyceridaemia (triglycerides 16422 mg/dl), and acute oedematous non‑lithiasic pancreatitis. Plasma exchange was initiated at admission and reduced triglycerides concentration to less than half. Two additional sessions of plasma exchange in the subsequent days, associated with pharmacological treatment of hypertriglyceridaemia, achieved a normal triglycerides level at discharge and allowed a favourable clinical evolution with rapid resolution of the pancreatitis.
Key‑Words: Hypertriglyceridaemia; pancreatitis; plasmapheresis.
INTRODUCTION
Acute pancreatitis (AP) is a potentially life‑threatening inflammatory condition of the pancreas, which at may affect the peripancreatic tissues as well as other organs.
The determination of its aetiology is essential for its management. Severe hypertriglyceridaemia (HTG), usually defined as serum triglycerides (TG) concentration above 1000mg/dl, is the third most common cause of acute pancreatitis, right after alcohol abuse and gallstone disease1‑4.
It has been described as the subjacent cause in up to 10% of cases of AP in general, but in more than half of these cases occurs during pregnancy1,3,4.
Hypertriglyceridaemic acute pancreatitis (HTGP) seems to have a worse outcome than pancreatitis of other aetiologies and the outcome depends on our ability to rapidly reduce serum TG levels1,4,5. Plasma exchange (PE) may be helpful due to its ability to efficiently and rapidly remove TG from plasma.
CASE REPORT
We report a case of a 41‑year‑old male who resorted to the Emergency Department of our hospital because of complaints of nausea, biliary vomiting and precordial discomfort caused by effort over the last two days, followed by strong abdominal pain located at the right upper quadrant on the day of admission. His past medical history included high alcohol intake around 200g per day, diabetes mellitus diagnosed 5 years before which was considered as secondary to a previous pancreatitis of unknown aetiology, and obesity (BMI 30).
Patient compliance with the medical follow‑up and medication was very poor, and omeprazole, 20 mg daily, was the only drug taken of his prescribed medication.
There was no previous available lipid profile or history of treatment with cholesterol or TG‑lowering medication. Relevant family history included presumed ischaemic heart disease, as this was his mother and brothers cause of death at the age of 65 and 50, respectively.
On physical examination at admission he was apyretic, non‑icteric, blood pressure was 153/102 mmHg, pulse was 116 bpm and oxygen saturation was 99% on pulse oximetry. The abdomen examination was significant for tenderness in the right upper quadrant, with no other abnormalities detected. Respiratory, cardiovascular and neurologic examinations were unremarkable.
Laboratory studies at admission showed severe hypertriglyceridaemia (triglycerides 16,442 mg/dl), elevated low‑density lipoprotein cholesterol (LDL cholesterol) (LDL cholesterol 528 mg/dl), high amylase (amylase 102 U/L) and high lipase (lipase 317 U/L).
Other relevant laboratory results at the time of admission and reference range levels are listed in Table I.
Posterior workup showed uncontrolled diabetes (HbA1c 9%). An abdominal computerized tomography (CT) was performed and showed acute non‑lithiasic Oedematous pancreatitis, which in this context was considered as hypertriglyceridaemia‑induced.
This patient was admitted to the Intermediate Care Unit, and started on a fasting regimen. Given the severity of HTG, a decision was made to start PE treatment immediately.
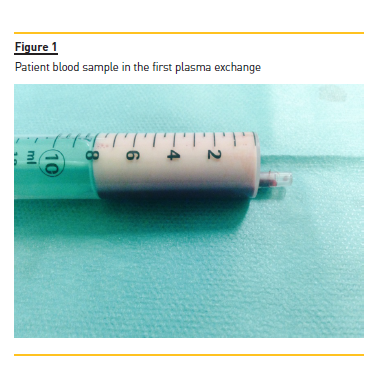
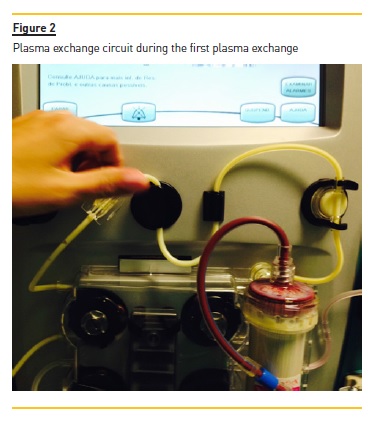
A temporary right femoral venous catheter was placed with no complications and he underwent the first PE treatment on the day of admission. A double membrane filtration device was used and albumin 5% was used as the replacement fluid. The volume treated was correspondent to one plasma volume and the duration of session was around 3 hours. The procedure was complicated by interference of HTG with the machine sensors and by the clotting of two filters due to the high plasma viscosity (Figs. 1 and 2), requiring high doses of unfractionated heparin. After the first session, his TG levels were more than halved from 16,455 to 7,845 mg/dl.
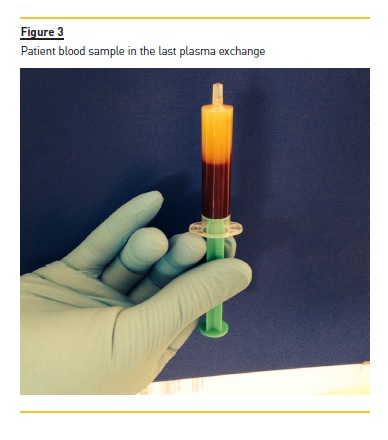
Two additional PE sessions were performed in the subsequent two days (Fig. 3). Triglycerides levels were 1,452 mg/dl after the second PE treatment, and 394 mg/dl after the third treatment. Additional treatment included gemfibrozil 600 mg twice daily, and diabetes control with insulin and diet intervention. A normal TG level (TG 158 mg/dl) was achieved at discharge (12 days after admission) and a complete recovery from his acute pancreatitis was seen. There were no PE‑related complications.
At discharge the patient was medicated with gemfibrozil 600 mg twice a day, atorvastatin 40 mg once a day and long‑acting insulin. At follow‑up, as an outpatient, one month later he was asymptomatic and his TG and pancreas enzymes were within the normal range.
DISCUSSION
The importance of apheresis treatments has been increasing in nephrology practice6. Plasma exchange is considered a safe and efficient procedure to rapidly reduce serum TG levels in patients with severe HTGP, with potential for improving the outcome. While there has been an increasing use of PE in patients with severe HTG (TG > 1000mg/dl) and AP6‑8, some clinicians still give preference to conservative treatment9. When assessing the value of PE in the treatment of hypertriglyceridaemic pancreatitis, one should take into account the fact that triglyceride levels decrease spontaneously with conservative treatment, and that the two treatments have not yet been compared in a controlled trial.
Causes of hypertriglyceridaemia may be categorized into genetically based (primary) and secondary disorders due to other diseases. Primary disorders include familiar congenital hyperlipidaemia, familial hypertriglyceridaemia, familial dysbetalipoproteinaemia, and familial chylomicronaemia syndrome10. Secondary disorders include uncontrolled diabetes mellitus, alcohol abuse, pregnancy and specific medications10. The extremely elevated levels of serum TG, his past medical history of previous pancreatitis of unknown cause andhis family history of ischaemic heart disease at young age, all point to a potential primary cause of HTG. However, there were other evident contributing factors, such as uncontrolled diabetes (HbA1c was 9.4%) and abusive alcohol intake (200g daily).
There is no clear threshold for which HTG is associated with AP. However, as high levels of chylomicrons are needed, severe HTG is generally defined by levels greater than 1000 mg/dl. As chylomicrons increase almost linearly with increasing TG, higher levels of TG correlate with a higher risk of AP11. The pathophysiology behind HTGP is, however, still a matter of debate. According to the most common theory, TG hydrolysis by pancreatic lipase causes a local release of a toxic amount of free fatty acids, which are capable of damaging acinar cells.
Additionally, there is an elevation of chylomicrons in the pancreatic capillaries, leading to capillary congestion and consequently to ischaemia and acidosis. Finally, large amounts of pancreatic secretions containing active pro‑enzymes, such as trypsinogen are released after direct cellular damage and duct block, culminating in the autodigestive process of pancreatitis1,4,7. Considering this sequence of events, it seems very logical that the outcome can be improved by a rapid removal of the causative agent, the TG (and more importantly the chylomicrons), and that treatment should be performed as soon as possible, theoretically before the pancreas autodestruction has become irreversible.
General treatment of HTGP is obviously of the utmost importance, including fasting and low‑calorie infusion, intensive intravenous hydration and analgesic treatment in the acute setting, followed by an oral fat‑free diet as pain disappears and intestinal transit normalizes1,3.
Standard therapeutic measures are based on lipid‑lowering agents, such as gemfibrozil, fenofibrate and niacin1,3,4. Control of other causative metabolic disturbances, such as diabetes mellitus, as in the case of our patient, is also imperative1.
Plasma exchange, on the other hand, is an extracorporeal process, that can be carried out as either an emergent or programmed procedure, and is very efficient in rapidly lowering TG levels and potentially ameliorating the course of HTGP. However, other potential beneficial mechanisms of action have been described3,4,12. This procedure may be able to remove proteases and pro‑inflammatory cytokines as well as reduce hyperviscosity. Additionally, if fresh frozen plasma is infused, then lipoprotein lipase and apolipoprotein C‑II might enhance the endogenous lipolysis, but its potential benefit remains unproven.
Case reports and anecdotic case series have been reported, but no randomized controlled trials (RCT) are available. Given the lack of RCT, currently it is unknown whether PE improves mortality and morbidity in all cases of HTGP. Some centres only use this technique in severe AP12, while others treat patients presenting with low APACHE II scores13,14. Chen et al. did not find PE to have a benefit effect on mortality when compared with conservative treatment15. To be noted, however, those authors admit there might have been a delay in the initiation of treatment, so there is a possibility that an earlier treatment may be necessary to achieve better results. Besides the uncertainty about efficacy, availability issues and high costs are sometimes limitations to the use of this therapy.
Currently, the American Society for Apheresis guidelines classified the use of PE for the treatment of HTGP as a category III indication, meaning that its benefit is not yet proven and that decision‑making should be individualized16. The Clinical Practice Guidelines recommend plasma exchange performed daily for 1 to 3 days depending upon the patient course and TG level16. The goal is to achieve TG concentrations lower than 500 to 1000 mg/dl.
One single PE session can reduce serum TG levels by 49 to 80%1,4,12. However, some patients need two or more sessions to reduce TG to below 1000mg/dl1,4.
Yeh et al. showed that a single plasma exchange treatment decreased TG by 66.3%, while a second session was able to decrease serum TG by 83.3%17. In their study, the average procedure duration was 2 hours and 10 minutes. They argued that short sessions of around 2 hours duration were associated with a lower transmembrane pressure of the PE filter and, thus, a greater TG clearance was obtained. However, the study found no relationship between the number of sessions and patient outcome.
Our patient presented with extremely high TG concentration and PE was very efficient, with the first treatment reducing TG levels by 52% and achieving a reduction of 97.6% at the third PE treatment.
The outcome may be influenced by the timing of apheretic treatment. Some authors have suggested that PE should be performed within the first 24 hours after admission for a maximal reduction in morbidity and mortality, while others have included patients 24 to 72 hours after admission1,4,16. Early initiation of PE treatment may improve the outcome by limiting the extent of organ damage, given the process of autodigestion typical of acute pancreatitis, but not all studies found a difference in morbidity and mortality when comparing an earlier with a later initiation of treatment1,12,13,16. Interestingly, in a recent series of 11 cases of HTGP treated with PE, patients who died (3 cases) had lower pre‑treatment TG concentrations than the ones who survived. A possible interpretation of these results is that lower TG levels may represent a delay in reaching the hospital and starting the treatment12, as TG concentration usually drops progressively as the patient fasts when the complaints of pancreatitis‑related symptoms start.
Additional controversy exists when discussing technical details of the PE technique. Both centrifugal and double membrane filtration have been used to treat HTGP. The hyperviscous plasma observed in severe HTG represents a special challenge. Centrifugal methods were found to promote a greater removal, because of the tendency of the TG to clog the pores of the filters2,16.
Gubensek et al. reported that in 3.7% of the procedures the plasma filter had to be replaced due to increased transmembrane pressure and in 1% the filter clotted and the procedure had to be restarted14. Another advantage of a centrifugal technique is the fact that lower blood flow rates are needed allowing treatment via peripheral veins, and thus avoiding the need for a central venous access, which is essential in the case of the membrane filtration technique. In our case, as described earlier in this article, we used a double membrane filtration technique and a higher dose of heparin was necessary in the first treatment to overcome the problem of high plasma hyperviscosity associated with very high TG levels. With regard to centrifugal and double membrane filtration techniques, a retrospective study recently compared the two modalities in 10 patients with hypertriglyceridaemic acute pancreatitis and suggested both techniques to be equally effective5. Therefore, in most cases, the decision on which technique should be chosen can be based only on its availability.
Heparin use as anticoagulant for the procedure may have advantages considering its ability to release lipoprotein lipase, which could enhance TG reduction16.
However, some studies have used citrate as anticoagulant with similar TG reduction and a recent observational study suggested a significantly reduced mortality using citrate anticoagulation as compared with heparin anticoagulation13,16. These studies, due to their observational nature and methodological problems, have a high risk of bias.
There is also no data to recommend one replacement fluid over another. Most studies have used 5% albumin, while some have used fresh frozen plasma (FFP) with the argument that this could enhance TG removal as FFP contains lipoprotein lipase16. When 5% albumin is used there is a progressive loss of coagulation factors.
Thus, patients bleeding risk increases, especially as the initial treatment requires intensive anticoagulation to avoid system clotting. There is some evidence suggesting that FFP during or after PE might be helpful in the correction of dyslipidaemia in patients with the rare genetically proven Apo CII or LCAT deficiency, but the use of FFP has also the disadvantage of higher infection risk5. Recently, Zeitler et al. suggested 5% albumin as the first choice replacement fluid5.
As previously referred, HTG is the cause of AP in 50% of cases during pregnancy, which is a rare but dangerous complication in those women affected. Fibrates are not recommended because of the paucity of data in this setting, and they may be harmful to the foetus. Yet, plasma exchange prior to or during an acute episode of hypertriglyceridaemic acute pancreatitis can quickly and safely lower the levels of TG and improve symptoms in this population3,18. Plasma exchange has also been suggested as a preventive measure in the treatment of recurrent pancreatitis in patients with severe HTG19.
In conclusion, PE has been used to treat hypertriglyceridaemic acute pancreatitis being effective for acute reduction of severe HTG. This study also suggested that PE may be effective for rapidly reducing TG levels in a patient with severe hypertriglyceridaemia and acute pancreatitis. Of note, triglyceride levels decrease spontaneously with conservative treatment, and PE has not yet been compared with conservative treatment in a controlled trial using clinical outcomes as endpoints. Therefore, further investigation is required to assess the benefit of PE in this setting.
References
1. Valdivielso P, Ramirez‑Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med 2014; 25(8):689‑694. [ Links ]
2. Stefanutti C, Julius U. Treatment of primary hypertriglyceridemia states – General approach and role of extracorporeal methods. Atheroscler Suppl 2015; 18:85‑94. [ Links ]
3. Stefanutti C, Labbadia G, Morozzi C. Severe hypertriglyceridemia‑related Acute pancreatitis. Ther Apher Dial 2013; 17(2):130‑137. [ Links ]
4. Edwald N, Kloer HU. Treatment options for severe hypertriglyceridemia (SHTG): the role of apheresis. Clin Res Cardio Suppl 2012; 7:31‑35. [ Links ]
5. Zeitler H, Balta Z, Klein B, Strassburg C. Extracorporeal treatment in severe hypertriglyceridemia‑induced pancreatitis. Ther Apher Dial 2015; 19(4):405‑410 [ Links ]
6. Izquierdo‑Ortiz MJ, Abaigar‑Luquin P. [Severe hypertriglyceridemia. Treatment with plasmapheresis]. Nefrologia 2012; 32(3):417‑418. [ Links ]
7. Castro FS, Nascimento AM, Coutinho IA, Alcazar FR, Mugayar Filho J. [Plasmapheresis as a therapeutic approach for hypertriglyceridemia‑induced acute pancreatitis]. Rev Bras Ter Intensiva 2012; 24(3):302‑307. [ Links ]
8. Costantini N, Mameli A, Maronglu F. Plasmapheresis for preventing complication of hypertriglyceridemia: a case report and review of literature. Am J Ther 2014: Epub ahead of print. [ Links ]
9. Uysal E, Acar YA, Gokmen E, Kutur A, Dogan H. Hypertriglyceridemia induced pancreatitis (chylomicronemia syndrome) treated with supportive care. Case Rep Crit Care 2014; 2014:767831. doi: 10.1155/2014/767831. [ Links ]
10. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an uptodate. J Clin Gastroenterol 2014; 48(3):195‑203. [ Links ]
11. Coca‑Prieto I, Valdivielso P, Olivecrona G, et al. Lipoprotein lipase activity and mass, apoliprotein C‑II mass and polymorphisms of apolipoproteins E and A5 in subjects with prior acute hypertriglyceridemic pancreatitis. BMC Gastroenterol 2009; 9:46. [ Links ]
12. Ramirez‑Bueno A, Salazar‑Ramirez C, Cota‑Delgado F, Torre‑Prados MV, Valdivielso P. Plasmapheresis as treatment for hyperlipidemic pancreatitis. Eur J Intern Med 2014; 25: 160‑163. [ Links ]
13. Gubensek J, Buturovic‑Ponikar J, Romozi K, Ponikvar R. Factors affecting outcome in hypertriglyceridemic pancreatitis treated with plasma exchange. PLoS One 2014; 9(7):e102748. [ Links ]
14. Gubensek J, Buturovic‑Ponikar J, Marn‑Pernat A, et al. Treatment of hyperlipidemic acute pancreatitis with plasma exchange: a single‑center experience. Ther Apher Dial 2009; 13(4): 314‑317. [ Links ]
15. Chen JH, Yeh JH, Lai HW, Liao CS. Therapeutic plasma exchange in patients with hyperlipidemic pancreatitis. World J Gastrenterol 2004; 10(15):2272‑2274. [ Links ]
16. Schwartz J, Winters JL, Padmanabhan A,
17. Yeh JH, Chen JH, Chiu HC. Plasmapheresis for hyperlipidemic pancreatitis. J Clin Apher 2003; 18(4):181‑185. [ Links ]
18. Zheng Y, Hu W, Wang J, Hu W, Liu C. Plasmapheresis for the treatment of hypertriglyceridemia-induced severe acute pancreatitis in pregnancy: it could be a good choice. Int J Colorectal Dis 2015; 30(10):1443‑1444. [ Links ]
19. Kadikoylu G, Yukselen V, Yavasoglu I, Coskun A, Karaoglu AO, Bolaman Z. Emergent therapy with therapeutic plasma exchange in acute recurrent pancreatitis due to severe hypertriglyceridemia. Transfus Apher Sci 2010; 43:285‑289. [ Links ]
Cátia Cunha
Serviço de Nefrologia (Nephrology Department),
Centro Hospitalar de Vila‑Nova de Gaia/Espinho,
Rua Conceição Fernandes
4434‑502
Vila Nova de Gaia, Portugal.
Tel.: 351 917223425
Disclosure of potential conflicts of interest: None declared
Received for publication: Sep 21, 2016
Accepted in revised form: Jan 26, 2016














