Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.4 Lisboa dez. 2016
ORIGINAL ARTICLE
Multiple myeloma and high cut-off haemodialysis: On the right track for better outcomes?
Teresa Chuva1, José Maximino1, Joselina Barbosa2, Paulo Santos1, Sandra Silva1, Ana Paiva1, Jorge Baldaia1, Teresa Santos1, Alfredo Loureiro1
1 Nephrology Department, Instituto Português de Oncologia do Porto, Rua Dr. António Bernardino de Almeida. 4200-072 Porto, Portugal
2 Department of Medical Education and Biomedical Simulation, Faculty of Medicine of the University of Porto, Al. Prof. Hernâni Monteiro, 4200-319, Porto, Portugal.
ABSTRACT
Background: Acute kidney injury secondary to cast nephropathy is a common complication of multiple myeloma. Extracorporeal light chain elimination by high cut-off haemodialysis has been described as an adjuvant to effective chemotherapy to limit free light chain toxicity. The purpose of this study was to evaluate the impact of high cut-off haemodialysis and bortezomib-based chemotherapy on renal function recovery and overall survival in a cohort of patients with multiple myeloma and dialysis-dependent acute kidney injury. Methods: We did a historical cohort study of patients with multiple myeloma and dialysis-dependent acute kidney injury presenting to our Centre between the 1st January 1999 and 31st March 2013. Results: Forty-six patients were included, with a median age of 68 (56-73 Y) years old. Twenty-four per cent recovered renal function. Patients submitted to high cut-off haemodialysis had a significantly higher probability of renal function recovery (OR = 11.5; 95% IC: 1.0 to 126.5). Seventy-two per cent of the patients died. The median survival rate was 20 months and overall 1-year survival rate was 58.3%. Male sex was associated with worse overall survival (HR = 4.9; 95% CI: 2.0-12.3). Renal function recovery decreased the risk of death (HR = 0.24; 95% CI: 0.07-0.80) as compared with those who remained on dialysis. The use of HCOH had no influence on the risk of death. Conclusions: Adding high cut-off haemodialysis to the novel anti-myeloma agents was independently associated to better renal outcomes in patients with multiple myeloma and dialysis-dependent acute kidney injury. However, our confidence in these results is hampered by the observational nature of the study, and by the small sample size and imprecise estimates of effect. Randomized controlled trials addressing this issue are urgently needed.
Key-Words: Acute kidney injury; chemotherapy; haemodialysis; multiple myeloma.
INTRODUCTION
Multiple myeloma (MM) is a plasma cell dyscrasia often associated with renal involvement, most commonly presenting as acute kidney injury (AKI) and proteinuria1.
Depending on the definition criteria, renal impairment is found in 20-50% of the patients at presentation2-4,1,5,6 and in further 25% within 2 months of the diagnosis6. Ten per cent have renal insufficiency severe enough to require dialysis7. A number of studies have also shown that patients with renal impairment are most likely to experience greatly increased morbidity and mortality compared with patients with normal kidney function2,4,5.
It is believed that severe AKI secondary to MM is mostly a consequence of direct exposure of renal tubules to the large amounts of free light chains (FLC) produced by a malignant plasma cell clone1. These large amounts of FLC overwhelm the absorptive capacity of the proximal tubules and are prone to precipitate with the Tamm-Horsfall protein, leading to cast formation and, thus, to luminal obstruction, interstitial inflammation and progressive fibrosis8,9.
Treatment strategies usually focus on three main approaches: correction of reversible risk factors (dehydration, hypercalcaemia, nephrotoxicity, other), prompt initiation of chemotherapy (CT) targeting the malignant clone and removal of FLC from the plasma.
Earlier studies using plasma-exchange (PE) to remove FLC from the plasma of patients with MM and AKI have given inconsistent and even contradictory results. To date, there is no convincing evidence that PE improves clinical outcomes in this setting10,11.
Indeed, PE only removes intravascular FLC in significant amounts. Once a session is completed, there is a rapid redistribution of FLC from the extra-vascular into the intra-vascular compartment and circulating levels rapidly return to baseline. Effective removal can only be achieved by extended sessions, allowing the redistribution of extra-vascular FLC into the intravascular compartment and subsequent removal. Yet, while the extended use of PE might be effective, its use is limited by clotting and deproteination problems.
Furthermore, while conventional haemodialysis (HD) is unable to remove FLC because of the small pores of dialysers membranes, dialysers with very large pores have been used in a number of studies with promising results. Pilot data have shown that high cut-off haemodialysis (HCOH) reduces the levels of serum FLC and improves renal function (RF) in patients with MM associated AKI, when combined with effective CT12-15.
Concurrently with the emergence of the aforementioned innovative dialysis techniques, the introduction of novel CT agents has widened the options available to treat MM patients with impaired RF16-18. Currently, the association of the proteasome inhibitor bortezomib with high-dose dexamethasone is usually considered as the most effective initial treatment for the majority of such patients18.
Altogether, new treatment approaches, such as the use of HCOH and bortezomib-based CT seem to have brought encouraging improvements to the management of MM-induced AKI, but this suggestion has been based in a small number of pilot studies that addressed this particular issue14,15. Thus, questions remain as to whether the use of HCOH represents an added benefit to the latest CT regimens, and namely whether the use of HCOH is able to improve clinical outcomes.
The aim of this study was to assess the benefit of using HCOH and bortezomib-based CT in renal function recovery (RFR) and overall survival in comparison with earlier treatments, in a cohort of patients with MMdependent AKI.
METHODS
Study population
We did a historical cohort study of patients with multiple myeloma and dialysis-dependent acute kidney injury presenting to our Centre between the 1st January 1999 and 31st March 2013.
Data collection included basic demographic details, type of myeloma, treatments received and clinical outcomes.
MM patients were included in the study independently of whether MM was primary diagnosed, relapsed or refractory.
Data and study outcomes
Forty-six patients were included with presumed myeloma-induced renal failure, after exclusion of other aetiologies. Twenty-four hour proteinuria, urine and serum protein electrophoresis, immunofixation and determination of free light chains were available in all patients but renal biopsy was not performed.
Chemotherapy regimens were determined by the hospital haematologist and included different therapeutic agents during the time range of this study, according to the current guidelines. Patients on bortezomib in some point of their treatment were recorded, as well as those who underwent haematopoietic stem cell transplantation (HSCT). The latter represented patients who were subject to transplantation and had subsequent relapsed myeloma.
Nine patients, presenting from 2010-2013, were eligible for extracorporeal serum FLC removal with HCOH: five were treated with Gambro Theralyte HCO membranes, three with Fresenius EMiC®2-filters and one patient was treated with both filters. Due to its lower molecular weights, patients with kappa light chains were treated with Fresenius EMiC®2-filters.
Five other patients underwent renal replacement therapy during the same time frame, but HCOH could not be used, since these expensive dialysers were not available at the time.
Extracorporeal therapy was undertaken with a blood flow of 200 ml/min and a dialysate flow of 300 ml/min, with adequate heparin and ultrafiltration according to clinical needs. Eight hours extended FLC removal haemodialysis was performed using human albumin solution as the replacement fluid in the patients in whom Theralyte filters were used. Magnesium, calcium and potassium were supplemented as required.
High cut-of haemodialysis was performed, initially, on 4 consecutive days, followed by every other day for another 4 sessions. Standard on-line haemodiafiltration (HDF) was continued as clinically indicated.
No direct technical complications were reported. There were, however, minor technical constraints. Due to logistical issues, sequential laboratorial monitoring of free light chain levels was not adequately performed and variations of the levels prior to and after HCOH could not be accurately interpreted. The primary clinical outcomes were death and RFR.
The latter was defined as independence from renal replacement therapy as determined by the supervising nephrologist.
This study was approved by the Ethics Comittee of the Instituto Português de Oncologia of Porto.
Statistical analysis
All data analyses were undertaken using the Statistical Package for Social Sciences version 22.0 for Windows.
Overall survival was defined as the time from the beginning of dialysis to death from any cause or last contact.
Patients who were still alive were censored at last followup date. Continuous variables were expressed as the median and percentile 25 (p25) and percentile 75 (p75) and categorical variables were expressed as absolute (N) and relative (%) frequencies. For further data analyses, continuous variables were categorized.
For comparison among groups, Chi-square test or Fishers exact test were used for categorical variables and Mann-Whitney test for continuous variables.
To identify variables associated with recovery renal function, variables that showed significance in univariate analysis were further investigated by multiple logistic regression. The strength of association was assessed by odds ratio (OR) and its respective confidence interval (CI) of 95%.
A Kaplan-Meier estimate was used to calculate the median survival time and survival rate. The log-rank test was performed to compare survival curves.
Factors associated with prolonged survival were analysed using multivariate Cox regression analysis.
The results are expressed as hazard ratios with 95% CI. The proportional hazards assumption was checked visually using Schoenfeld residuals.
RESULTS
Baseline characteristics
A total of 46 patients with MM and dialysis-dependent AKI were admitted to our Centre during the study period.
The median age at the beginning of dialysis was 68 (56- 73) years old and 57.4% were male.
Patients were divided into two categories, according to the year of initiation of renal replacement therapy (RRT): before and after 2010, when bortezomib became more widely used and HCOH-treatment was available.
Thirty-two patients (69.5%) were treated before the year 2010, of which only six (18.8%) used CT regimens comprising bortezomib (p < 0.001). None was submitted to HCOH (p < 0.001). They were comparable in the remaining aspects.
Nine patients were treated with HCOH. All of them were also treated with bortezomib regimens. The baseline characteristics of the study population are depicted in Table I.
Predictive factors for renal function recovery
Eleven patients (23.9%) recovered renal function and 63.6% of these were treated with HCOH. The group treated after 2010 had a significantly higher RFR rate than the group treated before 2010 (57% vs. 9%, p = 0.001) (Fig. 1).
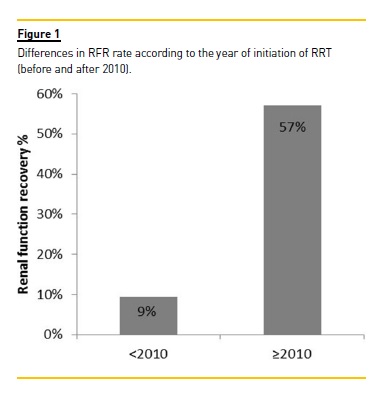
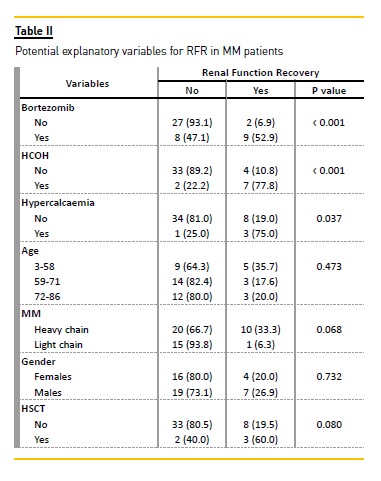
Using univariate analysis, three potential factors significantly increased the probability of RFR in the whole cohort: CT regime with bortezomib (p < 0.001), use of HCOH (p < 0.001) and the presence of hypercalcaemia (p = 0.037) (Table II).
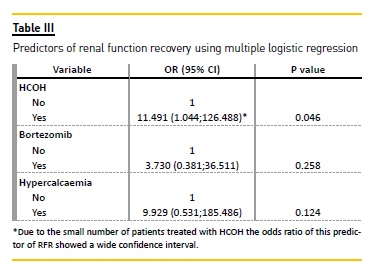
Using multiple logistic regression, the only significant predictor of RFR was the use of HCOH. Patients submitted to HCOH had a significantly higher probability of recovering renal function than patients who underwent conventional HDF (OR = 11.491; 95% IC: 1.044-126.488).
Patients survival
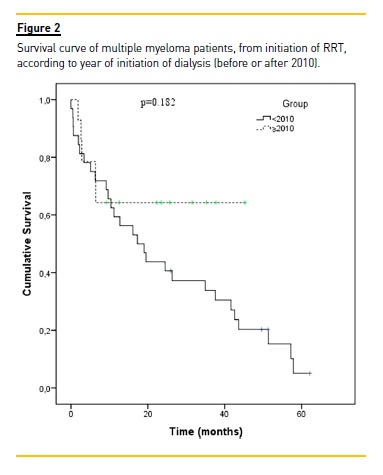
The median duration of follow-up for patients initiating RRT before and after 2010 was 18 months (range, 0 to 62) and 17 months (1 to 45), respectively. Among the former, 28 (87.5%) patients died, three (9.4%) were still alive at the end of follow up and one (3.1%) was lost to follow-up. Among the latter, four (28.6%) patients died and 10 (71.4%) were still alive at the end of follow up.
The median survival rate for patients treated before and after 2010 was 24.6 months and 30.5 months, respectively (p = 0.182). The survival curves of the two cohorts (initiating RRT before and after 2010) are shown in Fig. 2.
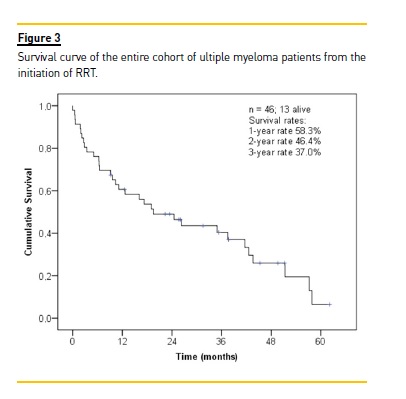
The median follow-up of the entire cohort was 18 months (range, 0 to 62). Thirty-three (71.7%) patients died, one (2.2%) was lost to follow-up and 13 (28.3%) were still alive at the end of follow-up. The median survival rate was 20 months and overall 1-year survival rate was 58.3% (Fig. 3).
In univariate analysis (Fig. 4), RFR (p < 0.001) and gender (p = 0.012) were significant predictors of overall survival. Age, type of light chain, use of HCOH, treatment with bortezomib, haematopoetic stem cell transplantation (HSCT) and the presence of hypercalcaemia were not associated with overall survival (Fig. 4).
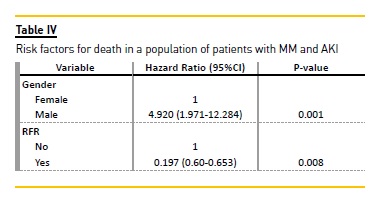
Using multivariate Cox regression, male sex was associated with an increased risk of death (HR = 4.920; 95% CI: 1.971-12.284), while RFR decreased the risk of death (HR = 0.244; 95% CI: 0.074-0.802) as compared with those remaining on dialysis (Table IV). The use of HCOH had no influence on the risk of death.
DISCUSSION
Dialysis-dependent renal insufficiency secondary to cast nephropathy is a frequent complication of MM and is associated with poor survival. Interventions that prevent kidney failure or those that favour renal recovery are, thus, of potential benefit. It is believed that to achieve such renal recovery a rapid reduction in serum concentrations of immunoglobulin free light chains is needed. Reducing the exposure of the kidneys to light chains might be one of the main therapeutic goals.
Recently, extracorporeal light chain elimination by HCOH has been described as an adjuvant therapy to CT to further improve renal outcome.
In this study, we analysed the clinical outcomes of a historical cohort of patients with MM and dialysisdependent AKI evaluating the prognostic impact of the use of HCOH and of bortezomib-based CT in RFR and overall survival. Sixty-nine point five per cent of our patients were treated before the year 2010, using mostly CT regimens, which did not include the use of bortezomib or HCOH.
Our results showed that patients treated with HCOH for extracorporeal light chain removal have a greater chance of RFR, even after adjustment for several risk factors. Renal function recovery was observed in a total of 23.9% patients, the majority of whom (63.6%) treated with HCOH. These results seem to be consistent with those of other studies published over the last few years, which found better outcomes associated with the introduction of new therapeutic agents and techniques [REFERENCES?].
Earlier published studies showed a rate of RFR of 26-29%2,5 in patients with MM induced AKI. Yet, according to recent studies, these results have improved dramatically with the use of new drugs, ranging from 63 to 80%, as has been reported by Ludwig et al.17, Matsue et al.19 and Kastritis et al.20.
However, these studies had severe limitations, such as the small sample size (8-12 patients with dialysisdependent AKI).
Recent studies evaluating the effect of the use of HCOH, as an add-on to CT, have reported an RFR of 63-77.8%14,15. However, these studies had a high risk of bias, resulting from the small sample size (these studies included 19 to 67 patients) and methodological problems.
Unexpectedly, our results did not find a significant association between bortezomib-based CT and RFR, thereby excluding the concurrent benefit of new CT regimens. However, the use of HCOH was associated with improved RFR and, in the subgroup of patients on extended HD, as much as 77.8% recovered renal function (RF), which is consistent with the aforementionedobservations.
In patients with MM and renal failure, the reported median survival ranges from 3.5 to 33 months1,2,5,21,22.
The median survival of 20 months observed in our patients is within this range. In this study, survival does not seem to be affected by age, type of light chain, use of HCOH, treatment with bortezomib, HSCT and presence of hypercalcaemia. However, our results suggest it may be influenced by gender and RFR. The independence from dialysis predicted a prolonged survival, while male sex conferred a poorer outcome.
The protective effect of RFR has usually been explained by data suggesting that MM patients on dialysis are frequently undertreated and do not receive optimal chemotherapy for the fear of side effects23,24. Additionally, they are often not considered for and are not able to benefit from HSCT25.
While several studies have shown that male sex is a risk factor for the development of MM4,26, its correlation with survival is controversial27,28. We were unable to explain this association and one might speculate whether it could be the result from distinct underlying characteristics of the male and female population that were not evaluated in our analysis.
Age is frequently pointed out as a prognostic factor, but this was not the case in our cohort4,5,7,22.
The lack of correlation between the use of HCOH and survival suggests that, possibly, some of the patients or of the multiple myeloma characteristics, which were not influenced by the treatment with HCOH, were responsible for the increased survival observed in the cohort after 2010 as compared with that before 2010.
We are well aware of important limitations to our study, namely the fact that it was a single centre study with a small sample size and a small number of events.
Due to the small sample size, the association between HCOH and RFR showed a wide confidence interval and, thus, an imprecise effect estimate, limiting the strength of the conclusions. Also, renal biopsy was not performed, meaning that, in some of the subjects, cast nephropathy may not be the cause of AKI, despite clinical exclusion of other aetiologies of renal failure. This fact may have influenced the response to therapy.
Another limitation is the fact that patients with newly diagnosed, relapsed or refractory MM were not distinguished.
Thus, distinct disease status might, by itself, justify a distinctive prognosis. And, finally, as previously reported, due to technical laboratory difficulties, monitoring of plasma free light chain levels during each dialysis session (pre- and post- HCOH) was not available for all cases, which limited its interpretation and, thus, this data was not analysed.
In summary, use of HCOH certainly appears to be a promising procedure that may increase RFR. However, more research is required to determine its precise role in the management of cast nephropathy.
Addressing this question, there is currently an ongoing prospective European multi-centre randomized controlled trial, led by Dr. Hutchinson, the European Trial of Free Light Chain Removal by Extended Hemodialysis (EuLITE)29.
References [ Links ]
2. Bladé J, Fernández-Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 1998;158(17:1889–1893. [ Links ]
3. Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma – A demographic study of 1353 patients. Eur J Haematol 1994;53(4):207–212. [ Links ]
4. Abbott KC, Agodoa LY. Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol 2001;56(3):207–210. [ Links ]
5. Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol 2000;65(3):175–181. [ Links ]
6. Irish AB, Winearls CG, Littlewood T. Presentation and survival of patients with severe renal failure and myeloma. QJM 1997;90(12):773–780. [ Links ]
7. Sharland A, Snowdon L, Joshua DE, Gibson J, Tiller DJ. Hemodialysis: An appropriate therapy in myeloma-induced renal failure. Am J Kidney Dis 1997;30(6):786–792. [ Links ]
8. Santostefano M, Zanchelli F, Zaccaria A, Poletti G, Fusaroli M. The ultrastructural basis of renal pathology in monoclonal gammopathies. J Nephrol 2005;18(6):659–675. [ Links ]
9. Huang ZQ, Sanders PW. Biochemical interaction between Tamm-Horsfall glycoprotein and Ig light chains in the pathogenesis of cast nephropathy. Lab Invest 1995;73(6):810–817. [ Links ]
10. Cserti C, Haspel R, Stowell C, Dzik W. Light-chain removal by plasmapheresis in myelomaassociated renal failure. Transfusion 2007;47(3):511–514. [ Links ]
11. Clark WF, Garg AX. Plasma exchange for myeloma kidney: cast(s) away? Kidney Int 2008;73(11):1211–1213. [ Links ]
12. Hutchison CA, Cockwell P, Reid S, et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol 2007;18(3):886–895. [ Links ]
13. Sinisalo M, Silvennoinen R, Wirta O. High cut-off hemodialysis and bortezomib-based therapy to rescue kidneys in myeloma-dependent cast nephropathy. Am J Hematol 2012;87(6):640. [ Links ]
14. Heyne N, Denecke B, Guthoff M, et al. Extracorporeal light chain elimination: high cutoff (HCO) hemodialysis parallel to chemotherapy allows for a high proportion of renal recovery in multiple myeloma patients with dialysis-dependent acute kidney injury. Ann Hematol 2012;91(5):729–735. [ Links ]
15. Hutchison CA, Heyne N, Airia P, et al. Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant 2012;27(10):3823–3828. [ Links ]
16. Chanan-Khan AA, Kaufman JL, Mehta J, et al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood 2007;109(6):2604–2606. [ Links ]
17. Ludwig H, Drach J, Graf H, Lang A, Meran JG. Reversal of acute renal failure by bortezomibbased chemotherapy in patients with multiple myeloma. Haematologica 2007;92(10):1411–1414. [ Links ]
18. Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol 2010;28(33):4976–4984. [ Links ]
19. Matsue K, Fujiwara H, Iwama K, Kimura S, Yamakura M, Takeuchi M. Reversal of dialysisdependent renal failure in patients with advanced multiple myeloma: single institutional experiences over 8 years. Ann Hematol 2010;89(3):291–297. [ Links ]
20. Kastritis E, Anagnostopoulos A, Roussou M, et al. Reversibility of renal failure in newly diagnosed multiple myeloma patients treated with high dose dexamethasone-containing regimens and the impact of novel agents. Haematologica 2007;92(4):546–549. [ Links ]
21. Torra R, Bladé J, Cases A, et al. Patients with multiple myeloma requiring long-term dialysis: presenting features, response to therapy, and outcome in a series of 20 cases. Br J Haematol 1995;91(4):854–859. [ Links ]
22. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78:21–33. [ Links ]
23. Goldschmidt H, Lannert H, Bommer J, Ho AD. Multiple myeloma and renal failure. Nephrol Dial Transplant 2000;15(3):301-304. [ Links ]
24. Boesler B, Czock D, Keller F, et al. Clinical course of haemodialysis patients with malignancies and dose-adjusted chemotherapy. Nephrol Dial Transplant 2005;20(6):1187–1191. [ Links ]
25. Lee C-K, Zangari M, Barlogie B, et al. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant 2004;33(8):823–828. [ Links ]
26. Tsakiris DJ, Stel VS, Finne P, et al. Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: an ERA-EDTA Registry study. Nephrol Dial Transplant 2010;25(4):1200–1206. [ Links ]
27. Conté LG, Figueroa MG, Lois VV, et al. Mieloma múltiple en Chile. Características clínicas y sobrevida. Rev Med Chil 2007;135(9):1111–1117. [ Links ]
28. Ludwig H, Durie BGM, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood 2008;111(8):4039–4047. [ Links ]
29. Hutchison CA, Cook M, Heyne N, et al. Eur opean trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE): a randomised control trial. Trials 2008;9:55. [ Links ]
Teresa Chuva
Rua Henrique Lopes de Mendonça, 253 H31
4150-396 Porto
Tel.: +351 916251999
Fax: 225084012
E-mail: m.teresa.chuva@gmail.com
Disclosure of conflicts of interest: None declared
Received for publication: Sep 10, 2015
Accepted in revised form: Jan 28, 2016














