Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.4 Lisboa dez. 2016
CASE REPORT
Type II mixed cryoglobulinaemia due to hepatitis C virus infection: The role of new direct-acting antivirals in a kidney transplant recipient
Rui Abreu1, Patrícia Neto1, Luís Oliveira1, Presa Ramos2, Teresa Morgado1
1Nephrology Department, Centro Hospitalar Trás-os-Montes e Alto Douro, Vila Real, Portugal2Hepatology Department, Internal Medicine, Centro Hospitalar Trás-os-Montes e Alto Douro, Vila Real, Portugal
ABSTRACT
Hepatitis C virus (HCV) infection remains prevalent in chronic kidney disease (CKD) patients. In a posttransplant patient, it can increase the risk for several complications such as transplant glomerulopathy and cryoglobulinaemia.
We describe a 69 year-old woman with chronic kidney failure secondary to autosomal dominant polycystic kidney disease (ADPKD) on haemodialysis since January 1989. She had HCV infection (genotype 1b) diagnosed within the dialysis period. Liver biopsy revealed signs of chronic hepatitis. Ten years later, she underwent a deceased donor kidney transplant. She maintained normal kidney allograft function and subnephrotic proteinuria.
In July 2015, she was hospitalized with necrotic ulcers in both legs. Laboratory findings revealed type II cryoglobulinaemia and low complement levels. HCV viraemia was high. Cutaneous biopsy showed a leukocytoclastic vasculitis. It was decided to treat cryoglobulinaemia and HCV infection with new direct-acting antivirals agents (DAAs) sofosbuvir/ledipasvir during 12 weeks. Skin ulcers improved and HCV RNA was undetectable after 4 weeks of treatment. Patient showed good tolerance for drug regimen. Proteinuria increased to nephrotic range after DAAs initiation. Donor-specific antibodies class I and II were negative and a kidney allograft biopsy demonstrated features of focal segmental glomerulosclerosis. Exacerbation of proteinuria in our patient could be a possible adverse effect of DAAs therapy, rarely reported in other cases.
Keywords: cryoglobulinaemia, direct-acting antiviral agents, HCV infection, kidney transplant recipient, sofosbuvir/Ledipasvir
INTRODUCTION
HCV infection remains prevalent in chronic kidney disease (CKD) patients1. The frequency of chronic HCV infection among renal transplant recipients is influenced by various factors, including blood transfusion, history of previous transplantation, type and duration of renal replacement therapy2. Liver biopsy is essential in the evaluation of liver disease in kidney transplant recipients because clinical and biochemical findings may underestimate its severity1. Despite HCV infection in end-stage renal disease tending to have an indolent course, HCV RNA titers usually increase and liver disease may progress after kidney transplantation as a result of immunosuppression3,2. HCV infection in kidney transplant recipients has a negative impact on patient and graft survival compared to those not infected4. However, kidney transplant is still the best renal replacement therapy option for these patients and represents a better chance of survival than remaining on haemodialysis4.Mixed cryoglobulinaemia syndrome is a common complication induced by HCV infection.2 The pathogenesis seems to be related to deposition of immune complexes in the glomerulus.3 The major clinical manifestations include palpable purpura, kidney disease, arthralgia, weakness and peripheral neuropathy2. Renal disease is present in 20% of patients at presentation of cryoglobulinaemia with variable nephrological syndromes such as microscopic haematuria, mild proteinuria, nephrotic syndrome or acute glomerulonephritis2. Diagnosis of cryoglobulinaemia is based on clinical, histopathological and laboratory findings such as cryoglobulin and low complement levels.2 The general therapeutic approach in cryoglobulinaemia syndrome includes two main principles: treatment of underlying disease and immunosuppression, depending on the severity of disease. Within the last few years, the treatment of HCV infection has completely changed. Interferon-based anti-HCV therapy is no longer recommended in kidney transplantation due to the high risk of acute rejection.1 Ribavirin is usually avoided as monotherapy because of its adverse effects such as haemolytic anaemia2.Direct-acting antivirals agents (DAAs) have been approved for treatment of HCV infection in CKD patients. Their most common mechanisms are inhibition of NS3/4A protease, NS5B polymerase and NS5A viral replication complex4. These new therapeutic regimens are characterized by shorter duration of treatment and better infection cure rates of more than 90%4.
Despite recent approval in treatment of HCV infection, experience with new-generation DAAs in kidney allograft recipients is low. Thus, the aim of this case report is to show our experience in treatment of cryoglobulinaemia, a frequent complication of HCV infection, with new DAAs in a kidney allograft recipient.
CASE REPORT
We describe the clinical case of a 69 year-old woman, with chronic kidney failure secondary to an autosomal dominant polycystic kidney disease (ADPKD) on haemodialysis since January 1989. After 10 years of haemodialysis, she developed severe hyperparathyroidism, for which underwent subtotal parathyroidectomy, and dialysis-related amyloidosis that was surgically corrected.
She had other comorbidities such as hypertensive complication of pregnancy that led to a preterm delivery; arterial hypertension treated with four antihypertensive drugs including ACE inhibitors; heart failure class II/III NYHA; paroxysmal atrial fibrillation and transient ischaemic attacks. Finally, she developed an HCV infection (genotype 1b) that was diagnosed within the dialysis period with fluctuating transaminases. A liver biopsy was performed in 1996 and revealed signs of chronic hepatitis (Knodell score: 2). She did not receive treatment with pegylated interferon or ribavirin during this time, due to absence of severe fibrosis or cirrhosis. In December 1999 she underwent a deceased donor kidney transplant with no major complications such as delayed graft function or episodes of acute rejection. The initial immunosuppression regimen was composed of cyclosporine, mycophenolate mofetil and corticosteroids. During the post-transplant period she suffered from osteoporosis and new-onset diabetes after-transplant. In January 2014, cyclosporine was changed to tacrolimus due to severe gum hypertrophy.
She had normal kidney allograft function (serum creatinine 0.8 mg/dL; ClCr 50 mL/min) and subnephrotic proteinuria (1.9 g/day). Serum cryoglobulins were detected from 2014 onwards, without signs of vasculitis.
In June 2015, a presumptive primary antiphospholipid syndrome was diagnosed due to the presence of IgM beta-2 glycoprotein antibody and previous thrombotic event (transient ischaemic attack and preterm delivery), so oral anticoagulation was initiated. One month later, she was hospitalized with necrotic ulcers in both legs (Figure 1a). She denied arthralgia, weakness or peripheral neuropathy symptoms. Laboratory findings revealed type II cryoglobulinaemia (276 ug/mL), low C3 and C4 levels, positive rheumatoid factor and negative anti-CCP. She maintained mild to moderate proteinuria and normal renal function. HCV viraemia was of 4960000 U/mL (log: 6.7) and transaminases were increased at that time aspartate aminotransferase and alanine aminotransferase (AST and ALT) of 96 and 87 U/L, respectively). Cutaneous biopsy was performed and leukocytoclastic vasculitis was confirmed. Despite severe vasculitic skin ulcers, it was decided to treat HCV infection with new direct-acting antivirals agents (DAAs) association sofosbuvir 400mg/ledipasvir 90mg, Immunosuppression such as cyclophosphamide or rituximab was decided against due to the high risk of hepatitis reactivation and liver disease progression.
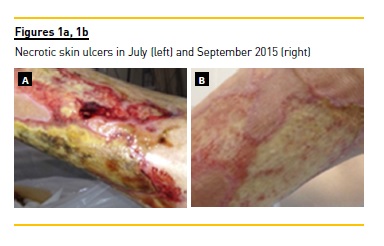
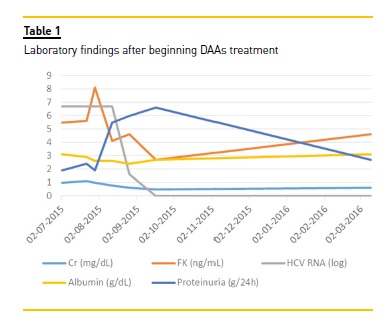
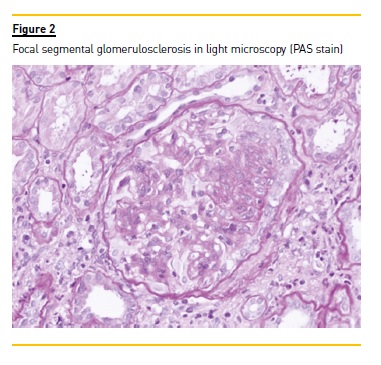
Necrotic skin ulcers improved gradually (Figure 1b). Transaminases normalized in the first week and HCV RNA reduced to 41 U/mL (log: 1.6) with only 2 weeks of therapy. After 4 weeks of treatment, viraemia was undetectable. Patient showed good tolerance to the drug regimen, and experienced epigastric pain only. Proteinuria increased to nephrotic range (6.6 g/day) after DAAs initiation, with no kidney allograft function exacerbation. Tacrolimus levels decreased slightly during antiviral therapy and dose adjustment was necessary (Table 1). After 12 weeks, IgM beta-2 glycoprotein antibody was repeated and its normalization excluded a primary antiphospholipid syndrome. Serum cryoglobulins were also negative in January 2016. Sustained virological response persisted at 12 and 24 weeks after treatment and transaminases remained normal during follow-up. In spite of clinical improvement and undetectable viraemia, nephrotic proteinuria persisted (4.0 g/day) after the antiviral treatment finished. Donor specific antibodies class I and II were both negative and a kidney allograft biopsy was performed. The histopathology result demonstrated features of focal segmental glomerulosclerosis, probably due to chronic allograft dysfunction (Figure 2). No morphologic evidence of transplant glomerulopathy was seen in light microscopy (PAS stain), such as double contours of glomerular basement membrane. Interstitial fibrosis and tubular atrophy were noted in 40% of the kidney.
Immunofluorescence showed absence of C4d or other deposition. ACE inhibitors were reintroduced and proteinuria decreased to values of less than 3.0 g/day.
DISCUSSION
HCV infection has been associated with deleterious effects in kidney allograft recipients.5 It can increase the risk of several complications, including liver disease, NODAT, glomerulonephritis, transplant glomerulopathy and cryoglobulinaemia6. This last entity usually has an insidious course but in some cases can lead to a lifethreatening condition. In our case, we found a low progression of systemic disease, within 2 years and only with cutaneous involvement. The patient had moderate and stable proteinuria (1.9 g/day) over the last few years, and for that reason an allograft biopsy was not performed. Treatment of cryoglobulinaemia syndrome includes two main strategies: immunosuppression or therapy against underlying disease. In this case, there was no rapidly progressive disease or severe organ threat to justify immunosuppressive therapy. On the other hand, interferon is generally not recommended in kidney transplant recipients due to the risk of allograft rejection and ribavirin can cause severe anaemia7. Currently, in consequence of the appearance of new-generation DAAs, sofosbuvir/ledipasvir were chosen and administered for 12 weeks. Only four weeks later, HCV RNA was undetectable. This fast response in kidney transplant recipients was seen in the Beinhardt et al study8. They demonstrated a slower response in haemodialysis patients, with 50% (5/10) having a detectable HCV viraemia at week 4, compared with 13% (1/8) in the post-kidney transplantation group8.
In the Kamar et al study, a rapid virological response was observed in 88%.5 Sustained virological response (SVR) in our patient persisted at 12 and 24 weeks after treatment. This efficacy was seen in the same Beinhardt et al study, in which 96% of patients achieved SVR at 12 and 24 weeks of follow-up8. However, only one patient was treated with sofosbuvir/ledipasvir. In the Lin et al study, 29.2% (7/24) patients received sofosbuvir/ledipasvir without ribavirin9.The overall rate of viral cure was 91% (SVR at 12 weeks)9. Better results were seen in the Sawinski et al study.10 They treated 20 kidney recipients, 88% infected with HCV genotype 1 such as our patient.10 The most frequent regimens used were sofosbuvir/simeprevir (45%) and sofosbuvir/ledipasvir (35%).10 All patients (100%) reached sustained virological response10.
Serum transaminases promptly normalized in our case after one week of treatment for HCV infection, as seen in the Eisenberger et al study11. Serum cryoglobulins also decreased from 276 ug/mL to undetectable range, six months later. Sise et al showed that cryoglobulin levels disappeared in four out of nine cases12.
In this case, the patient tolerated the antiviral treatment well. She reported only epigastric pain, probably due to suspension of omeprazole. Proton-pump inhibitors are described as reducing absorption of ledipasvir by increasing gastric pH8. Despite almost half (40%) of the kidney transplant recipients having chronic kidney disease in the Beinhardt et al study, 88% tolerated the full dose of sofosbuvir well8. Renal function remained stable during treatment in this case, in accordance with other studies such as that of Sawinski et al. A serum creatinine increase >0.25 mg/dL was reported in only 20% (4 patients) due to high tacrolimus levels (2) and anti-hypertensive drugs (2)10. Our patient needed a slightly dose adjustment in immunosuppression (increase of 1mg) throughout the antiviral treatment.
Similar conduct was seen in the last-mentioned study, with nearly half of the patients (45%) requiring calcineurin inhibitor dose change10. This variability can be explained by resolution of liver disease and enhanced metabolism of tacrolimus10. Unlike recent evidence, proteinuria increased to nephrotic range at the same time of DAAs administration in our case. In the Kamar et al study, six kidney allograft recipients were treated due to the presence of cryoglobulinemia and proteinuria5. Albuminuria maintained stable during follow-up5.
Similar findings were found in the Eisenberger et al study11. It is difficult to realize the cause for proteinuria exacerbation in our patient but we cannot exclude a possible adverse effect of DAAs association, rarely reported in other series. More studies and case reports are needed to better understand the safety of this newgeneration DAAs in kidney allograft recipients.
References
1. Ladino M et al. Hepatitis C virus infection in chronic kidney disease. J Am Soc Nephrol 2016 [ Links ]
2. Bunchorntavakul C et al. Management of patients with hepatitis C infection and renal disease. World J Hepatol 2015; 7(2): 213-225. [ Links ]
3. Burra P et al. Hepatitis C virus infection in end-stage renal disease and kidney transplantation. Transpl Int 2014; 27(9): 877-891. [ Links ]
4. Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol 2015; 11(3): 172-182. [ Links ]
5. Kamar N et al. Efficacy and safety of sofosbuvir-based antiviral therapy to treat hepatitis C virus infection after kidney transplantation. Am J Transplant 2016; 16(5): 1474-1479. [ Links ]
6. Kidney Disease: Improving Global, O. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int 2008; 73 (Suppl 109):S1-S99. [ Links ]
7. Belga S, Doucette KE. Hepatitis C in non-hepatic solid organ transplant candidates and recipients: a new horizon. World J Gastroenterol 2016; 22(4): 1650-1663. [ Links ]
8. Beinhardt S et al. DAA-based antiviral treatment of patients with chronic hepatitis C in the pre- and postkidney transplantation setting. Transpl Int 2016;29(9):999-1007 [ Links ]
9. Lin MV et al. Efficacy and safety of direct acting antivirals in kidney transplant recipients with chronic hepatitis C virus infection. PLoS One 2016; 11(7): e0158431. [ Links ]
10. Sawinski D et al. Successful treatment of hepatitis C in renal transplant recipients with direct-acting antiviral agents. Am J Transplant 2016; 16(5): 1588-1595. [ Links ]
11. Eisenberger U et al. Successful treatment of chronic hepatitis C virus infection with sofosbuvir and ledipasvir in renal transplant recipients. Transplantation 2016 Aug 5. [Epub ahead of print] [ Links ]
12. Sise ME et al. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology 2016; 63(2): 408-417. [ Links ]
Rui Miguel Guimarães Abreu
Av. da Noruega, Lordelo
5000-508 Vila Real, Portugal.
Email: ruimigabreu@gmail.com
Potential conflicts of interest: none declared.
Received for publication: Aug 8, 2016
Accepted in revised form: Dec 26, 2016














