Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.2 Lisboa jun. 2017
CASE REPORT
Urinary tract infections under 24 months old: Is it possible to predict the risk of renal scarring?
Ana M. Miranda, Catarina Garcia, Vanda A. Bento, Sara Pinto
Paediatrics Department, Hospital Prof. Doutor Fernando Fonseca E.P.E., IC19, 2720‑276 Venteira, Amadora – Portugal
ABSTRACT
Background: Urinary tract infection is one of the most common bacterial infections in the first two years of life and it can lead to irreversible renal scarring. Renal scintigraphy is the gold standard method for detection of renal scars. The aim of our work was to revise the cases of pyelonephritis, detect the possible predictors for renal scarring and compare those results we would have obtained if we had followed current NICE guidelines. Methods: Retrospective analysis of all patients aged under 24 months evaluated in the paediatric department and diagnosed with pyelonephritis during a three‑year period. We excluded the cases in which no renal scintigraphy was performed. Results: Of the 59 children analysed, 50.8% were boys and 86.4% were under one‑year old. Escherichia coli was the predominant bacteria. Renal ultrasonography showed abnormal findings in 23 patients (39%). The incidence of renal scarring was 15.3%. Age, atypical urinary tract infection and abnormal renal ultrasonography seem to be correlated with risk of renal scarring, although the results were not statistically significant. C‑reactive protein level is significantly correlated with renal scarring risk (p=0.047). Working outside the NICE guidelines allowed us to catch 7 further renal scars. Conclusions: Its arguable if renal scintigraphy must be performed in all cases of pyelonephritis diagnosed in the first 24 months of life or only when there are other risk factors for renal scarring. Age, atypical urinary tract infection, C‑reactive protein level and renal ultrasonography results must be taken into account in the decision to perform renal scintigraphy in a child. More prospective studies with larger cohorts are needed.
Keywords: Child, renal scarring, renal scintigraphy, urinary tract infection.
INTRODUCTION
Paediatric Urinary Tract Infection (UTI) is a microbial inflammation of lower urinary tract (cystitis and urethritis), upper urinary tract (urethritis and pyelonephritis [PNA]) or both. UTI is one of the most common infections in children and symptoms may be nonspecific, mainly in children under five years old1.
The most important factors contributing to UTI are age and sex. In the first three months of life UTI is more common in males, after which the prevalence rate among females is higher. In full‑term neonates the rate of UTI is low (0.7%)1. In the literature, UTI prevalence in infants aged between two and 24 months with fever without any apparent cause is 13.4%2. The prevalence rate reported in girls aged one to five years of age is 1 to 3%, whereas few infections occur in boys in this age group1.
PNA can lead to transient and even permanent renal parenchymal abnormalities that are visible on dimercaptosuccinic acid renal scanning (DMSA). However, in recent data the true consequences of a post‑infectious renal scar (RS) are not clear. The evidence showing the association between childhood UTI and chronic kidney disease (CKD) in children with structurally normal kidneys is scarce and this association is likely weak. According to the literature, the most probable cause of CKD in children is not UTI but congenital structural abnormalities, renal dysplasia and hypoplasia. Nevertheless, it is hard to differentiate between post‑infectious RS and hypoplasia or dysplasia through image alone3,4.
The rate of post‑PNA RS is dependent on several risk factors, namely young age, female gender, urinary tract anomalies, presence of fever, delay of treatment, number of UTI episodes, laboratory indices of inflammation such as total white blood cell count (WBC) and C‑Reactive Protein (CRP) value, and uncommon uropathogens (non‑Escherichia coli)5,6. Although large‑scale studies have shown a low rate of renal injury after an episode of PNA, the evaluation of renal scarring was not the primary objective of these studies5. The true risk of renal scarring after a UTI thus remains unknown.
The choice of imaging exams to perform is probably the most controversial decision in children with a PNA.
After a first PNA episode, some authors argue in favour of early imaging in order to detect congenital abnormalities that may predispose the child to additional persistent or recurrent infections and renal damage7,8. The noninvasive nature, lack of radiation, and relatively low cost of ultrasonography have made it an ideal initial screening tool in children after a first PNA episode7. Yet, according to some international guidelines, the criteria to perform renal and bladder ultrasound (RBUS) does not include every children with PNA9,10. DMSA is the elected method for detection of RS1. Limitations of this exam include its significant cost, exposure to radiation, possible need for patient sedation, and limited availability1. There is no consensus on who must undergo DMSA following a UTI.
Traditionally, all PNA in children younger than 24 months of age are investigated. However, recent international guidelines argue that most DMSA exams following a PNA are normal and DMSA use should not be generalized8‑10.
There are different criteria for performing this exam and there is no consensus on which children should undergo it8‑10.
Over‑limiting the prescription of DMSA means some RS will likely be missed and future consequences on renal scarring are not yet known.
The aim of the present study was to: 1) revise the cases of PNA; 2) detect the possible predictors for renal scarring in cases of PNA; 3) assess the consequences of a hypothetical scenario whereby the NICE guidelines for initial imaging strategies were followed instead of our current clinical practices for PNA.
SUBJECTS AND METHODS
Case review of clinical files covering a three‑year period (from January 2013 to December 2015) of children under 24 months with PNA evaluated at the paediatrics department of a Lisbon metropolitan area hospital.
UTI was defined as any proliferation of a single bacterial strain obtained through supra‑pubic approach, growth of 10,00 to 50,000 colony‑forming units (CFUs) per mL in a sample collected from bladder catheterization/supra‑pubic aspiration or at least 100,000 CFUs/mL in a sample obtained from a collecting bag8,10. All children included presented two cultures isolating the same microorganism; at least one obtained from bladder catheterization or supra‑pubic approach. We defined PNA based on a confirmed UTI as well as the presence of at least one of the following criteria of fever no less than 38.0°C or loin pain/tenderness9.
Atypical UTI was considered if one of the following was documented: isolation of a microorganism rather than Escherichia coli, presence of palpable abdominal mass, altered renal function, initial presentation as sepsis and also no improvement after 48 hours of appropriate antibiotherapy9.
Serum for laboratory investigations, including WBC and CRP, were taken in some patients, according to clinical criteria.
Empirical antimicrobial treatment followed the departments clinical protocol, adjusted according to the antibiogram of the uropathogen in each case.
Antimicrobial prophylaxis was prescribed according to our departments clinical protocol. In the first year of this study, we prescribed antimicrobial prophylaxis to all children with a first PNA and without sphincter control of the bladder or to children with uropathy, but in the last two years antimicrobial prophylaxis was only prescribed in cases of uropathy or recurrent UTI.
RBUS was performed in every patient at diagnosis. We collected information on the kidneys (number, size, location, parenchyma features), urinary tract (dilatation, duplicity, wall thickening), and bladder (ureterocele). All abnormal RBUS findings were recorded, including the antero‑posterior diameter of the renal pelvis no less than five millimetres (mm) and/or any grade of dilatation of the calyces or ureters irrespective of antero‑posterior diameter; pelvic or ureteral wall thickening; solitary or ectopic kidney; absence of cortico‑medullary differentiation; duplicated renal collecting system, and kidney size discrepancy. Renal pelvic wall thickening was defined as thickening measuring greater than one mm. Kidney size discrepancy was defined as kidneys dimension discrepancies greater than 10 mm.
DMSA was performed at least six months after the diagnosis in every patient. Renal asymmetry was considered as a difference in the analysis of renal quantitative activity equal to or superior than 10%. Renal scarring was defined as evidence of kidney focal or generalized areas of persistent diminished radioisotope uptake in DMSA.
Children with prenatal history of hydronephrosis, neurogenic diseases, history of urogenital or anorectal malformations and all those who did not undergo follow‑up with DMSA were excluded from the study.
Children with no clinical information on the first UTI were also excluded from the study. Children with urine samples collected only from a collecting bag were excluded. Children with a lower UTI were excluded from the study.
The NICE guidelines were used to determine the indications for DMSA. According to NICE guidelines, a DMSA should be performed only in the presence of an atypical or recurrent UTI9.
Data were gathered regarding demographic and clinical facts, laboratory and imagiology results, use of antimicrobial acute treatment and prophylaxis, type of follow‑up appointment and family history of renal disease.
Statistical analysis was performed using SPSS® 21.0 (SPSS, INC., Chicago, USA). For numerical variables with normal distribution, the mean and standard deviation (SD) were calculated. The median, minimum and maximum values were calculated for non‑normal distribution variables. The comparative analysis was performed using a chi‑square test or Fishers exact test for categorical variables. For continuous variables with normal distribution, the Student t‑test was performed and the Mann‑Whitney U test for continuous variables with non‑normal distribution. Data were analysed as to what the consequences would have been if the NICE guidelines had been applied to clinical use in this cohort, with regard to missed diagnoses.
RESULTS
Epidemiology
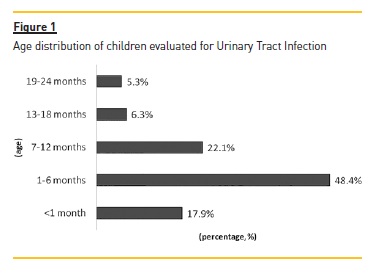
In the period analysed, there were 59 UTI episodes that met the inclusion criteria. There was a slight male predominance among the sample (50.8% were boys) and the median age was four months (range 13 days – 20 months). A total of 44.1% were aged equal to or under three months old and 86.4% were aged no more than twelve months. The distribution according to age group is represented in Figure 1. Only two children (3.4%) had reported familiar history of nephro‑urologic diseases.
Clinical findings and diagnostic exams
The median length of time between the beginning of symptoms and initiating antibiotherapy was one day (range 0 – 9 days).
Analysis with WBC and CRP were performed in 86.4% children. The mean leukocyte count value was 16.9±6.4 x109/mL and median CRP value was 6.81 mg/dL (range 0.29 – 27.81mg/dL).
All 59 children had two urine cultures isolating the same microorganism. Escherichia coli was the most common pathogen isolated in 53 cultures (91.5%) with one of these isolating an Extended‑Spectrum Beta‑Lactamase (ESBL) producing Escherichia coli. Proteus mirabilis was isolated in 3.4% children, followed by Klebisiella pneumoniae, Klebsiella oxytoca and Citrobacter koseri all in 1.7%.
Atypical UTI was documented in 7 (11.9%) children: four had isolation of microorganisms rather than Escherichia coli; two patients did not improve after 48 hours of appropriate antibiotherapy and one newborn that presented with sepsis. There was no difference between clinical, laboratory and ultrasound features in both groups with and without atypical UTI, except in the rate of admission. Children with atypical UTI had a smaller rate of admission after UTI diagnosis (2.8% vs 26.1%, p=0.011).
RBUS was performed in every patient either at admission or during the first day of therapy. In 36 (61.0%) children no alterations were documented. A total of 16 (27.1%) children had a renal pelvis anteroposterior (AP) diameter greater than five millimetres.
One (1.7%) children presented with pyelocalicial duplicity with ureterocele, and two (3.4%) had an ectopic pelvic kidney. Two children (3.4%) had kidney size discrepancies.
Poor corticomedullary differentiation was described in one child (1.7%) and also one (1.7%) child had renal pelvic wall thickening.
Antibiotic therapy
The majority of children (61.0%) required admission to the hospital for intravenous antibiotherapy whereas only 23 were discharged home with an oral antibiotherapy prescription. 94.4% of the admitted patients were less than 12 months old. The median age of the children discharged home was 9 months (range: 3 – 20 months); that of the children hospitalized was two months (range: 13 days – 20 months). 86.1% of the children hospitalized were started on intravenous antibiotherapy and later switched to oral therapy, while 5 (13.9%) performed the total course of therapy intravenously.
All children started empiric antibiotic therapy. 35 (59.3%) were started on monotherapy with either amoxicillin/clavulanic acid (50.8%), cefuroxime (3.4%) and three (5.1%) children that presented with fever, elevation of blood infectious parameters but no clinical symptoms suggesting UTI started ceftriaxone empirically.
The remaining 24 patients were treated initially with combined antibiotherapy either due to their age or clinical presentation at diagnosis. 5 (8.5%) children less than one month were started on combined therapy with cefotaxime and gentamicin; 18 (30.5%) were started on amoxicillin/clavulanic acid with gentamicin.
One (1.7%) child that presented with fever, grunting and no specific alteration in urinalysis at the rapid dipstick method performed at the emergency department was started on cefotaxime combined with ampicillin.
A total of 12 (20.3%) children were initially treated with empiric antibiotics that were later revealed to be resistant to the isolated bacteria. There were no immediate complications described in any case.
After completing therapy, prophylactic antibiotherapy with trimethoprim was initiated in 45.8% children.
This subgroup had a median age of three months (range: 14 days – 16 months).
Follow‑ up and complicationsAll children had follow‑up hospital appointments either with a paediatric nephrology expert (79.7%) or a general paediatrician (20.3%).
Renal DMSA scintigraphy was performed in every patient. Alterations were documented in 14 (23.7%): in five children (8.5%) there was a difference in renal activity equal to or superior than 10% but with no focal defects; in nine (15.2%) renal scarring was reported.
Children with renal scarring had a median age of seven months (range 1 – 20 months). No information about further follow‑up was available.
Evaluating children with documented renal scarring at scintigraphy, it is possible to compare several parameters present at diagnosis with children that did not develop this complication.
Concerning gender, there was no significant differences between boys and girls (13.3% vs 17.2%, p=0.478).
The group with RS tended to be of an older age (7 vs 4 months, p=0.147) with UTI frequently due to other bacteria than Escherichia coli (20% vs 14.8%, p=0.577), although not significantly. There was no difference in length of time between symptoms and initiation of antibiotherapy between the groups with and without RS (2.50 vs 1.00; p=0.261). There was no association between the development of renal scarring and the initial empirical therapy with a resistant antibiotic (8.3% vs 17.0%, p=0.409).
For WBC count at diagnosis, there was no significant difference between groups: WBC at diagnosis (16.90± 3.89 x109 /mL vs 16.92± 6.65x x109 /mL, p=0.995).
However, CRP level at diagnosis were significantly higher in the group with RS (11.15 mg/dL vs 5.44 mg/dL, p=0.047). Children that had alterations documented at the RBUS performed at diagnosis had more RS at follow‑up (17.4% vs 13.9%, p=0.495), although not significantly.
When comparing children that were discharged and did performed oral antibiotherapy with those who were hospitalized and received, at least initially, intravenous antibiotherapy, the first group had a tendency toward more RS that the latter (26.1% vs 8.3%, p=0.071). Finally, we found a tendency to RS in the group with atypical UTI (28.6% vs 13.5%, p=0.288), although this not significant. Table I illustrates the comparison between children with and without RS.
If we had followed 2007 NICE guidelines, we would have performed only seven DMSA (11.9%). In the group of non‑atypical UTI, the majority had no DMSA alternations (76.9% from a total of 52 cases). However, following 2007 NICE guidelines, we would have missed 12 DMSA alterations (85.7% of DMSA alterations) in children with non‑atypical UTI (including seven RS).
From those with an atypical UTI, 71.4% had a normal DMSA.
DISCUSSION
Acute PNA may sometimes lead to permanent RS with possible significant late clinical sequelae3. In our study, we detected RS after a first episode of upper UTI in 15.3% patients aged under 24 months old, which is consistent with the literature (7.2 – 58%)6,7,11‑21.
The wide range found in the literature results from heterogeneous patient populations differing widely in age, different diagnostic criteria for UTI, inclusion of hospitalized patients or hospitalized and ambulatory treated individuals, criteria and timing for performing DMSA and ultimately different familiar and personal background. Some studies excluded young infants aged less than two months old11,13,15‑19.
However, Chih‑Chuan Hsu et al compared a population of infants aged less than two months and a population aged two to 24 months old, both hospitalized with first febrile UTI, and found no differences in clinical characteristics, antimicrobial resistance, imaging findings, and clinical outcomes21.
The general findings of our study were compatible with the literature: upper UTI was predominant in children under 12 months old7,15,19 and Escherichia coli was isolated in almost 90% urine samples11,15,16,19‑21.
Two fifths of the analysed patients had abnormal RBUS7,15,19‑21.
According to earlier literature, young age, female gender, presence of fever, urinary tract anomalies, delay of treatment, number of PNA episodes, laboratory indices of inflammation such as total WBC count and CRP concentration, and uncommon uropathogens (non‑Escherichia coli) were correlated with the development of renal scars5,6. However, some recent studies have failed to show these correlations6,11,19.
Warren T. Snodgrass et al studied children aged 0 – 18 years old referred for urology assessment after diagnosis of febrile UTI and/or vesicoureteral reflux (VUR).
Their data report a higher likelihood of focal DMSA cortical defects in children older than one year old versus younger than one year of age (18% versus 6%, p=0.001), with each year of life increasing the odds of having focal DMSA defect(s) after upper UTI (OR=1.2, 95% CI=1.1 – 1.3)13. A similar association was found by Nader Shaikh et al that retrospectively studied children younger than six years with a first or second upper UTI episode (2 – 11 months: 4%; 12 – 35 months 7%; 36 – 72 months: 15,9%; p=0,003)17. In our report there was a tendency to a higher risk of RS with increasing age (7 vs 4 months, p=0.147), which is consistent with the literature. However, there are other studies that failed to detect association between age and risk of RS11,19,20.
Despite previous reports which stated that girls have a higher risk of focal renal defects, we found no association between gender and risk of RS (17.2% vs 13.3%, p=0.478). This is in agreement with other recent studies6,7,12,13,16,17,20.
In a meta‑analysis of nine studies of 1280 children with a first UTI, temperature of at least 39°C was associated with RS (p<0.01)12. In this report the grade of fever was not analyzed and this could be an important factor in the risk of RS, which limits the results.
There was no significant difference in the incidence of RS and delay in the initiation of antibiotic therapy (2.5 days vs 1 day, p=0.261), which is consistent with the literature11,12,16,19. However, in some reports, the risk of renal scarring was higher when there was a delay greater than 72 hours in starting antibiotherapy19,20.
Consequently, there seems to be no greater risk of renal scarring if the onset of antibiotic therapy is delayed until a definitive diagnosis is made in children under 24 months who present with fever and are otherwise well.
In our study, most patients diagnosed with UTI were admitted to the hospital (61%) and 94.4% of admissions were infants aged less than 12 months. Akshay Sood et al studied the rates of hospital admission of paediatric UTI (≤17 years) in the United States of America between 2006 – 2011. Children aged less than 12 months had an admission rate of 22.6% although they represent 57.9% of the total admissions22. The huge difference between our results and the previous study is probably explained due to different criteria of hospital admission and because this study also included cystitis. We found a tendency to a higher risk of RS among infants discharged home (26.1% vs 8.3%, p=0.071). We could not find any related data in the literature. This is probably related to at‑home non‑compliance with medical treatment but may also be associated with the fact that the children who are discharged home are mostly older that the ones who are admitted and in this group, as stated before, there was tendency toward more RS risk.
Previous studies reported a significant relation between WBC and CRP with renal scarring risk12,16,23.
A meta‑analysis that included children aged less than seven years old demonstrated that the measurement of serum procalcitonin (PCT) can provide considerable predictive value for the development of renal scars, and that this predictive capacity is better than that provided by either CRP or WBC count23. In this report, children with RS did not have higher WBC values at diagnosis (WBC count: 16.9 ± 3.89 x109 /mL vs 16.92± 6654x x109/mL, p=0.995). However, CRP level was significantly higher in children with RS (11.15 mg/dL vs 5.44 mg/dL, p=0.047). It is important to note that not all children performed laboratory analyses and this might influence the results. Since these laboratory parameters are almost routinely performed in children under 24 months old with febrile ITU, prospective studies must be carried out to confirm the relation described, which might have a future influence on follow‑up approach.
Although recent studies have identified a significant relation between RS and non Escherichia coli in urine cultures12,19, there are also some reports that do not show this association between the pathogen isolated and the risk of irreversible renal lesions. In our study, isolation of non Escherichia coli In urine seems to increase the risk of RS (20% vs 15%, p=0.579).
We obtained an empirical antibiotic resistance of 20.3% in our study and no difference was found in the RS risk and initiating antibiotic therapy with an antibiotic that the isolated bacteria was resistant to. This is probably explained by the fact that in cases of antibiotic resistance, the therapy was changed as soon as the urine culture results were known, on average in the first 48 hours of treatment.
The number of abnormal RBUS was tendentiously higher in patients with renal scaring (17.4% vs 13.9%, p=0.495), which is consistent with other studies7,12,14,16,19,21. According to the Nader Shaikh et al meta‑analysis, RBUS is an important predictor of RS, regardless of age, sex and clinical appearance12. Some current guidelines limited the use of RBUS after the first UTI to young children or to children with an atypical UTI, and following this approach it is likely that we will have missed some RBUS and DMSA alterations.
Finally, our study seems to reinforce the tendency of having RS within atypical UTI cases (28.6% vs 13.5%; p=0.288)9.
The follow‑up approach after a UTI episode in children is being questioned and a consensus has not been reached yet. Some investigators argue against limiting the image studies to specific subgroups, namely children aged less than six months or with atypical UTI, because it could lead to missing RS diagnosis with its possible clinical consequences15,18,24,25.
Still we must take into account the disadvantages of performing an exam such as a DMSA: costs, radiation exposure, possible need for patient sedation.
So, the question persists: how many useless imaging exams are justified to find one patient with renal scarring?
Its crucial to find risk factors for RS in order to limit the prescription of DMSA after an upper UTI. According to our study, atypical UTI, PCR concentration and abnormal RBUS seem to be important features that we must include in a DMSA prescription algorithm.
This study has several strengths. Data were collected from a single centre with a consistent strategy in managing children with UTI. We enrolled hospitalized and outpatient children aged up to 24 months with a first UTI without previous nephro‑urologic malformations.
We performed late DMSA and RBUS on all included patients. The study has also some limitations. Firstly, its retrospective nature limits our conclusions. Secondly, the sample size (59 infants and children) may be a major limitation. Thirdly, information about some important follow‑up information namely recurrent UTI was lacking. Besides, both RBUS and DMSA are exams with subjective results. Another limitation is not knowing the exact time of the onset of infection. The closest we were able to come was to document accurately the onset of symptoms. Moreover, blood collection was inconsistent, and, therefore, there was a substantial number of patients with missing laboratorial data. This issue is associated with a high risk of bias.
The optimal imaging algorithm for children with UTI remains unclear. The choice of performing a DMSA after a UTI must be individualized and not generalized to all.
We must take into account at least age, presence of atypical UTI, PCR level and RBUS result when choosing which exams to perform. Further prospective studies with larger cohorts are warranted to reach a consensual clinical guideline.
References
1. Schlager TA. Urinary tract infections in infants and children. Microbiol Spectrum 2016; 4:UTI‑0022‑2016. [ Links ]
2. Wani KA, Ashraf M, Bhat JA, Parry NA, Shaheen L, Bhat SA. Paediatric urinary tract infection: a hospital based experience. J Clin Diagn Res 2016; 10: SC04‑SC07 [ Links ]
3. Craig JC, Williams GJ. Denominators do matter: its a myth–urinary tract infection does not cause chronic kidney disease. Pediatrics 2011; 128: 984‑985 [ Links ]
4. Salo J, Ikäheimo R, Tapiainen T, Uhari M. Childhood urinary tract infections as a cause of chronic kidney disease. Pediatrics 2011; 128: 840‑847 [ Links ]
5. Becknell B, Schober M, Korbel L, Spencer JD. The diagnosis, evaluation and treatment of acute and recurrent pediatric urinary tract infections. Expert Rev Anti Infect Ther 2015;13:81‑90 [ Links ]
6. Lee YJ, Lee JH, Park YS. Risk factors for renal scar formation in infants with first episode of acute pyelonephritis: a prospective clinical study. J Urol 2012;187:1032‑1036 [ Links ]
7. Hung TW, Tsai JD, Liao PF, Sheu J‑N. Role of renal ultrasonography in predicting vesicoureteral reflux and renal scarring in children hospitalized with a first febrile urinary tract infection. Pediatr Neonatol2015;20:1‑7 [ Links ]
8. American Academy of Pediatrics. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011; 128: 595‑610 [ Links ]
9. National Institute for Health and Clinical Excellence (2007). Urinary tract infection in children: diagnosis, treatment and long‑term management. NICE guideline (CG54). Pediatrics 2011;128(3):595‑610 [ Links ]
10. González Rodríguez JD, Rodriguez Fernández LM. Infección de vías urinarias en la infancia. Protoc Diagn Ter Pediatr 2014;: 91‑108
11. Hewitt IK, Zucchetta P, Rigon L et al. Early Treatment of acute pyelonephritis in children fails to reduce renal scarring: data from the Italian renal infection study trials. Pediatrics 2008: 122:486‑490. [ Links ]
12. Shaikh N, Craig JC, Rovers MM et al. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta‑analysis with individual patient data. JAMA Pediatr 2014; 168: 893‑900 [ Links ]
13. Snodgrass WT, Shah A, Yang M et al. Prevalence and risk factors for renal scars in children with febrile UTI and/or VUR: a cross‑sectional observational study of 565 consecutive patients. J Pediatr Urol 2013 9: 1‑15 [ Links ]
14. Bush NC, Keays M, Adams C et al. Renal damage detected by DMSA, despite normal renal ultrasound, in children with febrile UTI. J Pediatr Urol 2015; 11: 126e1‑7 [ Links ]
15. Narchi H, Marah M, Khan AA, Al‑Amri A, Al‑Shibli A. Renal tract abnormalities missed in a historical cohort of young children with UTI if the NICE and AAP imaging guidelines were applied. J Pediatr Urol 2015; 11; 252.e1‑7 [ Links ]
16. Yılmaz S, Özçakar ZB, Şür EDK et al. Vesicoureteral reflux and renal scarring risk in children after the first febrile urinary tract infection. Nephron 2016;132:175‑180 [ Links ]
17. Shaikh N, Mattoo TK, Keren R et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr 2016; 170: 848‑854 [ Links ]
18. Scola C, Mutiis CM, Hewitt IK et al. Different guidelines for imaging after first UTI in febrile infants: yield, cost, and radiation. Pediatrics 2013;131:e665‑e671 [ Links ]
19. Azor BR, Fernández JMR, Cárdenas SS et al. Cicatrices renales en menores de 36 meses ingresados por pielonefritis aguda. An Pediatr 2017; 86: 76‑80 [ Links ]
20. Karavanaki KA, Soldatou A, Koufadaki AM, Tsentidis C, Haliotis FA, Stefanidis CJ. Delayed treatment of the first febrile urinary tract infection in early childhood increased the risk of renal scarring. Acta Paediatr 2017; 106: 149‑154 [ Links ]
21. Hsu CC, Tsai JD, MD, Ku MS et al. Antimicrobial resistance and diagnostic imaging in infants younger than 2 months old hospitalized with a first febrile urinary tract infection: a population‑based comparative study. Pediatr Infect Dis J 2016; 35: 840‑845
22. Sood A, Penna FJ, Eleswarapu S et al. Incidence, admission rates and economic burden of pediatric emergency department visits for urinary tract infection: data from the nationwide emergency department sample, 2006 to 2011. J Pediatr Urol 2015;11(5):246. e1‑8 [ Links ]
23. Leroy S, Fernandez‑Lopez A, Roya Nikfar R et al. Association of procalcitonin with acute pyelonephritis and renal scars in pediatric UTI. Pediatrics 2013: 131: 870‑879 [ Links ]
24. Lytzen R, Thorup J, Cortes D. Experience with the NICE guidelines for imaging studies in children with first pyelonephritis. Eur J Pediatr Surg 2011; 21:283‑286 [ Links ]
25. Ristola MT, Hurme T. NICE guidelines cannot be recommended for imaging studies in children younger than 3 years with urinary tract infection. Eur J Pediatr Surg 2015; 25: 414‑420 [ Links ]
Ana Catarina Margalha Miranda
Hospital Prof. Doutor Fernando Fonseca, E.P.E., IC19
2720‑276 Venteira, Amadora – Portugal
Email: ana.margalha.miranda@gmail.com
Disclosure of potential conflicts of interest: none declared
This research did not receive any specific grant from funding agencies in the public, commercial, or not‑for‑profit sectors.
Received for publication: Mar 23, 2017
Accepted in revised form: Jun 29, 2017














