Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.3 Lisboa set. 2017
CASE REPORT
Rivaroxaban-related nephropathy
Miguel Oliveira1, Carla Lima1, Mário Góis2, Helena Viana2, Fernanda Carvalho2, Sérgio Lemos1
1 Department of Nephrology, Centro Hospitalar Tondela-Viseu, Viseu, Portugal.
2 Department of Nephrology, Centro Hospitalar de Lisboa Central – Hospital de Curry Cabral, Lisboa, Portugal.
ABSTRACT
Novel oral anticoagulants (NOAC) are a group of drugs recently approved for the treatment of patients at high risk of arterial or venous thrombosis.
Unlike vitamin K antagonists, NOAC have a faster onset of action, less drug interactions and do not need frequent monitoring, making their prescription more convenient for physicians and patients.
However, both classes of drugs have the potential for nephrotoxicity. Anticoagulation-related nephropathy is a form of acute kidney injury caused by excessive anticoagulation, resulting in glomerular haemorrhage and tubular obstruction by red blood casts. It was first described with warfarin, but new cases have been reported with acenocumarol and dabigatran.
We report a case of a 82-year-old woman suffering from atrial fibrillation under rivaroxaban treatment with previous normal renal function, admitted to our unit with gross haematuria and acute kidney injury. A renal biopsy revealed typical features of anticoagulant-related nephropathy superimposed on chronic interstitial nephritis and hypertensive nephroangiosclerosis.
There was no recovery of renal function despite withdrawal of rivaroxaban and the patient started on chronic haemodialysis three months after initial episode.
To the best of our knowledge, this is the first case of a patient with a biopsy-proven anticoagulant nephropathy related to rivaroxaban. As novel oral anticoagulants are increasingly being prescribed and available data on their safety concerning renal toxicity are scarce, this report highlights the importance of a cautious prescription of these drugs and regular clinical and laboratory evaluation to avoid poor clinical outcomes.
Key-words: Acute kidney injury; Anticoagulant-related nephropathy; Novel oral anticoagulants; Rivaroxaban.
INTRODUCTION
Anticoagulant-related nephropathy (ARN) is a type of acute kidney injury in the setting of overcoagulation1.
ARN is a recent recognized entity that was originally described in patients receiving warfarin, and it was formerly called warfarin-related nephropathy (WRN)2.
However, recent data show that ARN may occur with other anticoagulants besides warfarin, such as acenocumarol3 and dabigatran, a direct thrombin inhibitor that belongs to a class of drugs recently approved for long-term anticoagulation, and called novel oral anticoagulants (NOAC)4.
In ARN, there is extensive intraglomerular haemorrhage with tubular obstruction by red blood cells (RBC) casts, causing acute kidney injury2.
Here we report a case of a female with previous normal function presenting macroscopic haematuria and acute kidney injury due to overcoagulation with rivaroxaban, a factor Xa inhibitor, whose kidney biopsy revealed tubular obstruction by RBC casts and acute tubular necrosis (ATN), confirming the diagnosis of ARN induced by rivaroxaban.
CASE REPORT
A 82-year-old woman with previous normal function (serum creatinine 1.0 mg/dL, estimated glomerular filtration rate using Chronic Kidney Disease Epidemiology Collaboration Equation – CKD-EPI – 52.4 mL/min/1.73m2) presented with 3 days of gross haematuria in April 2017.
She had a past medical history of arterial hypertension, type 2 diabetes, hyperlipidaemia, congestive heart failure and atrial fibrillation. Her daily medication included furosemide 40 mg id, amlodipine 10 mg id, perindopril 10 mg id, atorvastatin 10 mg id, long-acting insulin 20 IU and rivaroxaban 20 mg id (prescribed 2 months before).
Since the patient had been started on rivaroxaban, no assessment of renal function had been made.
On admission the patient´s blood pressure was 145/84 mmHg, pulse rate 74 beats/min and tympanic temperature of 36,7° C. She was euvolaemic. Further physical examination was unremarkable.
Laboratory findings were as follow: haemoglobin 9.9 g/dL with normal leucocyte and platelet counts; prothrombin time (PT) 25 seconds with international normalized ratio (INR) of 2.3; serum creatinine 5.0 mg/dL and serum urea 203 mg/dL with normal electrolytes; serum albumin 3.9 g/dL; urinalysis revealed mild proteinuria (560 mg/24 hour) and erythrocyturia (5900/μL). Xa activity was not evaluated.
Serum and urinary proteins electrophoresis showed no monoclonal component; serologies for human immunodeficiency virus, hepatitis B and C virus, antinuclear antibodies and anti-neutrophil cytoplasmic antibodies were negative. Complement was normal.
Renal ultrasound revealed normal-sized kidneys with preserved corticomedullar differentiation.
The patient underwent a kidney biopsy and light microscopy revealed 6 glomeruli, 3 of which were in global sclerosis. There was intense interstitial fibrosis, extensive inflammatory infiltrates (mononuclear cells and plasma cells) and tubular atrophy (Figure 1), occlusive RBC casts (Figure 2) with interstitial haemorrhage (Figure 3) and diffuse acute tubular necrosis. The arteries showed intense intima thickness (Figure 4). The immunofluorescence examination was negative for deposition of IgA, IgM, IgG, C1q, C3 and kappa and lambda light chains.
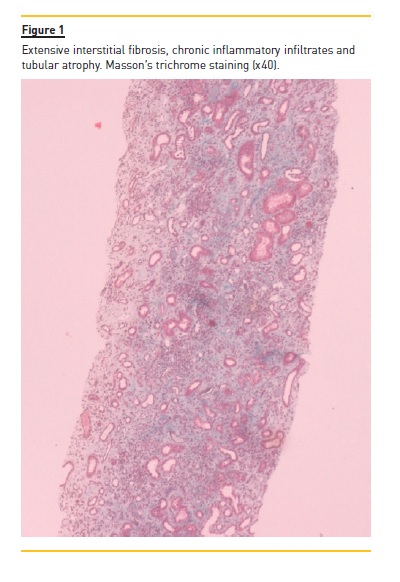
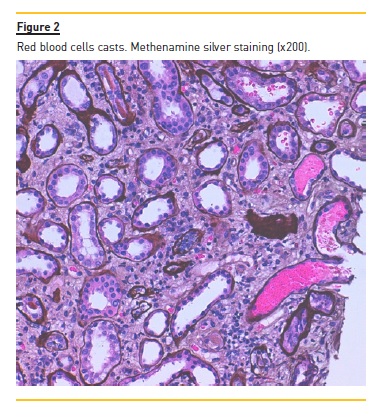
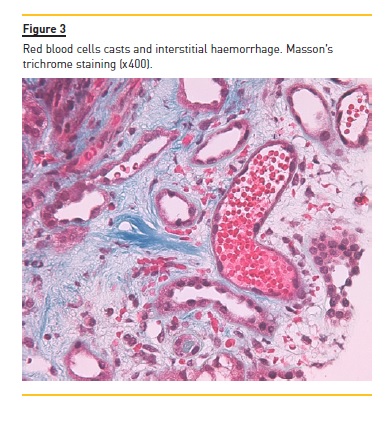
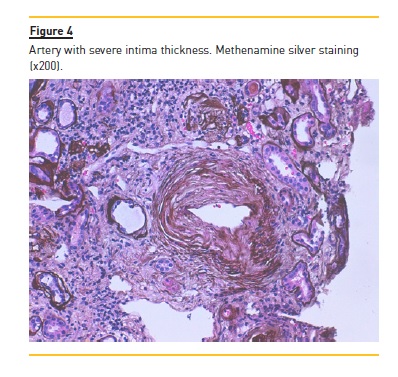
These histologic features led to the diagnosis of ARN, hypertensive nephroangiosclerosis and chronic interstitial nephritis.
The patient was asymptomatic and daily urinary output was preserved (>1.5 litre/day), without any electrolyte or acid-base disequilibrium. Her creatinine stable at 5.5 mg/dL. Rivaroxaban and perindopril were withdrawn and she was started on enoxaparin 60 mg/day and N-acetylcysteine 600 mg/day. She had no further episode of gross haematuria.
She was discharged and was under regular surveillance at our unit. The last clinical evaluation (July 2017) showed deterioration of kidney function (serum creatinine 10.1 mg/dL, serum urea 232 mg/dL) and the patient complained of symptoms of uraemia (nausea, anorexia), so she was started on chronic haemodialysis.
DISCUSSION
Vitamin K antagonists (VKA) such as warfarin and acenocumarol have been the mainstay of long-term anticoagulation over the last few decades5. Although they provide protection against thromboembolic events, VKA display an unfavourable pharmacokinetic profile, have a numerous list of drugs and nutritional interactions and require regular laboratory monitoring, reducing patient´s compliance with treatment, mainly in the elderly5,6.
Moreover, VKA can induce AKI, a new entity called ARN, which is defined as increase in serum creatinine without other obvious aetiology, in the setting of an INR > of 3.0, accompanied by microscopic or gross haematuria1,2,7.
This syndrome was first described in patients receiving warfarin, and was called warfarin-related nephrophaty8,9.
However, a few cases of ARN induced by acenocumarol3,10 have recently been reported. On this basis, regular assessment of kidney function and coagulation during the first three months of treatment is strongly recommended, and deterioration of renal function should immediately raise the hypothesis of ARN1.
NOAC were developed to overcome the disadvantages of VKA and there are now three drugs recently approved for long-term anticoagulation: these include a thrombin inhibitor (dabigatran), and two factor Xa inhibitors (rivaroxaban and apixaban)5. NOAC have a predictable pharmacokinetic and dynamics profile, and routine coagulation evaluation in not required5. Many clinical trials have shown non-inferiority of NOAC as compared to VKA, and no significant differences between NOAC and VKA with respect to major bleedings5,6. NOAC are alternative to VKA that are being increasingly precribed1, but some cases of ARN induced by dabigatran have been reported4,7,11,12.
Rivaroxaban was approved for clinical use in 2012 and has 66% renal elimination5; it is contra-indicated when creatinine clearance is < 15 mL/min and doses should be reduced if clearance is 15–49 mL/min13.
There are some cases of rivaroxaban-associated acute interstitial nephritis14, but none of ARN related to rivaroxaban.
The pathophysiological mechanisms underlying ARN are complex and imply disruption of glomerular filtration barrier with subsequent haemorrhage into Bowman´s space and renal tubules, causing obstruction and ischemia1,4. VKA decrease thrombin activity, and therefore there is underactivation of protease activated receptor-1 (PAR-1), a receptor for thrombin expressed in endothelial cells engaged in the regulation of endothelial functions, vascular permeability, leucocyte migration and adhesion4,7. Activation of PAR-1 is important for maintaining endothelial integrity, and reduction of thrombin levels induced by anticoagulants (VKA or dabigatran) enhances glomerular haemorrhage7,10,15.
There is some evidence that interstitial haemorrhage is also important in the development of ARN, increasing oxidative stress in the kidney4,7,11. Tubular cells are very sensitive to toxic heme proteins, which can further increase oxygen-radical formation, contributing to tubular and interstitial damage11,16.
Risk factors for development of ARN are previous CKD, age, arterial hypertension, diabetic nephropathy, glomerulonephritis and heart failure10.
On kidney biopsy, typical histological features of ARN are the presence of occlusive RBC casts, acute tubular necrosis and interstitial haemorrhage1,4.
Renal prognosis depends on the presence of glomerulosclerosis and the degree of tubulointerstitial fibrosis, and kidney function will stabilize or improve in the absence of these histological lesions10.
Treatment of ARN includes returning INR to a therapeutic range10, and there is evidence that N-acetylcysteine prevents acute kidney injury due to its antioxidant effect17.
To the best of our knowledge, this is the first case of a biopsy-proven of ARN secondary to rivaroxaban.
As mentioned above, ARN is defined as AKI without other plausible aetiology in the setting of an INR > 3.0 because it was first described in association with warfarin, but NOAC are also responsible for new cases of ARN. Although NOAC can interfere with the determination of PT and INR, these laboratory tests are not suitable for NOAC18, so the initially conceived definition of ARN may be no longer correct and should be further revised. While NOAC are widely prescribed drugs that have recently been approved for long-term anticoagulation, little is known about their safety with respect to renal function and kidney outcomes, as well as haemorrhagic complications. Emerging evidence that NOAC can have many bleeding complications is increasing4. Close monitoring of coagulation by determination of activity of target coagulation factors or the measurement of circulating drugs can actually be a useful tool in minimizing side effects, unlike what was first promoted5.
Our patient was at a very high risk of development of ARN, in light of her advanced age, absence of regular evaluation of renal function, medical history of diabetes, arterial hypertension, heart failure, treatment with angiotensin converting enzyme inhibitors, and unknown CKD as demonstrated by renal biopsy. Her kidney function was previously normal as a result of adaptive mechanism due to glomerular hyperfiltration, and it is likely to be overestimated with the reduced muscular mass common in the elderly. During aging, there is a drop in the glomerular filtration rate (GFR) and decreased creatinine production as a consequence of sarcopaenia, so serum creatinine does not necessarily increase with a fall in the GFR19. Our patient experienced rapid progression to end-stage renal disease due to reduction of the functioning tubules after gross haematuria, in the presence of severe signs of chronic damage secondary to arterial hypertension and aging found on renal biopsy (glomerulosclerosis, chronic interstitial nephritis, tubular atrophy and intima thickness).
In conclusion, ARN is an important clinical problem related to NOAC, with profound impact on morbidity and mortality. Screening for risk factors is essential to avoid poor renal outcomes, and previous CKD is the strongest risk factor for ARN. Particular attention should be given to older patients, even those with normal renal function, since the aging process determines loss of renal mass that is accompanied by adaptive mechanisms such as glomerular hyperfiltration, making these patients more vulnerable to the development of kidney disease. Estimating GFR in the elderly remains a difficult task and creatinine-based equations have their own limitations, with repercussions in the NOAC dose prescribed. More clinical data on and experience in haemorrhagic complications and renal adverse effects of NOAC, as well regular kidney function evaluation, are essential.
References
1. Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost 2016;14:461–467. [ Links ]
2. Brodsky SV. Anticoagulants and acute kidney injury: clinical and pathological considerations. Kidney Res Clin Prac 2014;33:174–180. [ Links ]
3. Clearly CM, Moreno JA, Fernández B, et al. Glomerular haematuria, renal interstitial haemorrhage and acute kidney injury. Nephrol Dial Transplant 2010;25:4103–4106. [ Links ]
4. Escoli R, Santos P, Andrade S, Carvalho F. Dabigatran-related nephropathy in a patient with undiagnosed IgA Nephropathy. Case Rep Nephrol 2015;2015:298261. [ Links ]
5. van Gorp RH, Schurgers LJ. New insights into the pros and cons of the clinical use of Vitamin K Antagonists (VKAs) versus Direct Oral Anticoagulants (DOACs). Nutrients 2015;7:9538–9557. [ Links ]
6. Halperin JL, Hankey GJ, Wojdila DM, et al. Efficacy and safety of ribaroxavan compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the ribaroxavan once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in fibrillation (ROCKET AF). Circulation. 2014;130:138–146. [ Links ]
7. Ryan M, Ware K, Qamri Z, et al. Warfarin-related nephropathy is the tip of iceberg: direct thrombin inhibitor dabigatran induces glomerular haemorrhage with acute kidney injury in rats. Nephrol Dial Transplant 2014;29:2228–2234. [ Links ]
8. Santos C, Gomes AM, Ventura A, Almeida C, Seabra J. An unusual cause of glomerular hematuria and acute kidney injury in a chronic kidney disease patient during warfarin therapy. Nefrologia 2013;33(3):400–403. [ Links ]
9. Brodsky SV, Nadasdy T, Rovin BH, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 2011;80(2):181–189. [ Links ]
10. Góis M, Azevedo A, Carvalho F, Nolasco F. Anticoagulant-related nephropathy in a patient with IgA Nephropathy. BMJ Case Rep 2017. doi:10.1136/bcr-2016-218748. [ Links ]
11. Moeckel GW, Luciano RL, Brewster UC. Warfarin-related nephropathy in a patient with mild IgA nephropathy on dabigatran and aspirin. Clin Kidney J 2013;6:507–509. [ Links ]
12. Kalaitzidis RG, Duni Anila, Liapis G, et al. Anticoagulant-related nephropathy: a case report and review of the literature of an increasingly recognized entity. Int Urol Nephrol 2017;49(8):1401–1407. [ Links ]
13. Hughes S, Szeki I, Nash MJ, Tachil J. Anticoagulation in chronic kidney disease patients – the pratical aspects. Clin Kidney J 2014;7(5):442–449. [ Links ]
14. Monahan RC, Suttorp MM, Gabreëls BA. A case of rivaroxaban-associated acute interstitial nephritis. Neth J Med 2017;75(4):169–171. [ Links ]
15. Krishna VN, Warnock DG, Saxena N, Rizk DV. Oral anticoagulants and risk of nephropathy. Drug Saf 2015; 38(6):527–533. [ Links ]
16. Moussavian MR, Slotta JE, Kollmar O, et al. Hemoglobin induces cytotoxic damage of glycine-preserved renal tubules. Transpl Int 2007;20:884–894. [ Links ]
17. Ware K, Qamri Z, Ozcan A, et al. N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am J Renal Physiol 2013;304:F1421–F1427. [ Links ]
18. Samama MM, Contant G, Spiro TE, et al. Laboratory assessment of rivaroxaban: a review. Thromb J 2013;11:11. [ Links ]
19. Garasto S, Fusco S, Corica F, et al. Estimating glomerular filtration rate in older people. Biomed Res Int 2014;2014:916542. [ Links ]
Miguel Oliveira, MD
Department of Nephrology, Centro Hospitalar Tondela-Viseu,
Avenida Rei D. Duarte, 3504-509, Viseu, Portugal.
E-mail: pmig.oliv@gmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: Aug 1, 2017
Accepted in revised form: Sep 6, 2017














