Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.1 Lisboa mar. 2018
ORIGINAL ARTICLE
Color doppler ultrasound assessment of juxta-anastomotic stenosis in radiocephalic arteriovenous fistulas: endovascular or surgical approach
Ana Pimentel1, Paulo Almeida2, Norton de Matos2, Luís Loureiro2, Gabriela Teixeira2, Duarte Rego2, Sérgio Teixeira2, Joaquim Pinheiro3, Isabel Fonseca4, Telmo Carvalho3, José Queirós3
1 Nephrology Department, Centro Hospitalar do Algarve – Faro, Portugal
2 Grupo de Estudos Vasculares (GEV) – Porto, Portugal
3 Vascular Access Centre, NephroCare Porto – Porto, Portugal
4 Hygiene and Epidemiology (EPI) Department and Instituto de Saúde Pública da Universidade do Porto – Porto, Portugal
ABSTRACT
Background: Juxta-anastomotic stenosis (JAS) is a common complication of radiocephalic arteriovenous fistulas. There is diverging data as to the best therapeutic approach being angioplasty or surgery. Pre-operative color Doppler ultrasound (CDU) is accurately used for initial assessment of the vascular access and follow-up monitoring. The aim of this study was to evaluate immediate and long-term results of endovascular versus open surgical intervention of juxta-anastomotic venous stenosis of forearm radiocephalic fistulas and to test if CDU assessment can be used to ameliorate preoperative strategy and long-term outcomes.
Methods: This retrospective cohort study included 63 patients with JAS radiocephalic fistulas referred to vascular access consultation. CDU was used to assess preoperative morphological, functional and hemodynamic stenosis characteristics and according to specific criteria, allocate patients to endovascular or surgical treatment.
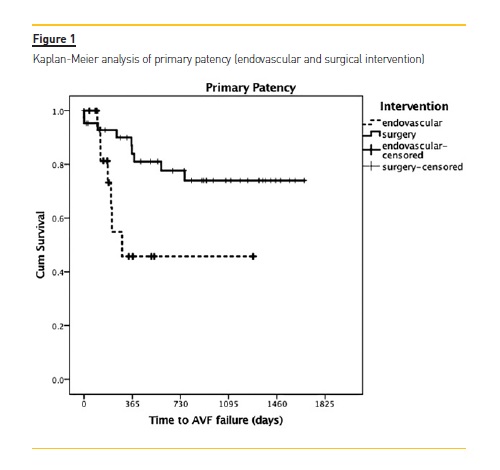
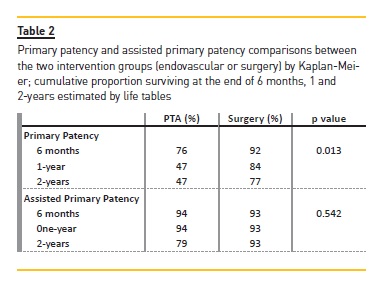
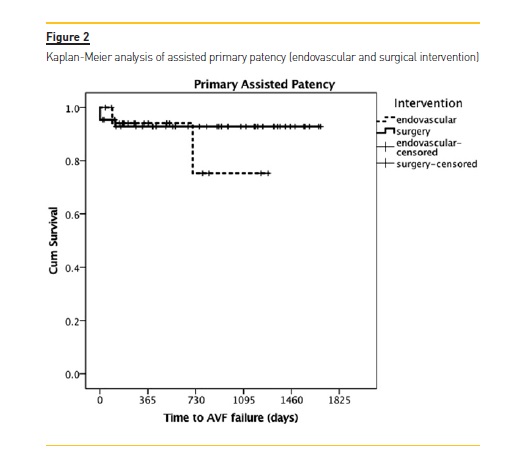
Results: Surgical revision was proposed in 68.2% of patients (N=43), namely the creation of a new proximal fistula (N=41), while angiographic evaluation was proposed in 31.7% of the cases (N=20). Mean follow-up time was 720±524 days with a maximum follow-up of 4.6 years. In the surgical group, primary patency was 92% and 84% at 6 and 12 months respectively, while in the endovascular group, it was 76% and 47% (p=0.013). There was no significant difference in the assisted primary patency between the interventional groups at 12 months: 94% in the endovascular vs. 93% in the surgical group (p=0.542).
Conclusion: Pre-operative CDU assessment of JAS and specific allocation criteria with an access-centered approach choosing the best option in each fistula allowed the correct diagnosis of the lesion, improved the global results of the treatment and optimized the financial resources by reserving PTA for selected cases where surgery could be more difficult with higher risk of access loss.
Keywords: Assisted primary patency, color doppler ultrasound, juxta-anastomotic stenosis, neo-anastomosis, percutaneous transluminal angioplasty. primary patency.
INTRODUCTION
Arteriovenous fistulas (AVF) commonly complicate with flow-limiting stenosis, an independent risk factor for AVF failure. Usually stenosis develops significantly earlier in radiocephalic AVF compared to other type of upper limb fistula1. The best approach to juxta-anastomotic stenosis (JAS) in radiocephalic AVF is still controversial.
Significant stenosis can be treated either with surgery or percutaneous transluminal angioplasty (PTA)2, the latter allowing better native venous preservation and being less invasive3.
Most importantly, early pre-emptive intervention, either PTA or surgical correction, results in lower incidence of AVF thrombosis4. The two procedures are considered equally valid, complementary for pre-emptive treatment of stenosis in radiocephalic AVF5. The choice depends on technical availability, local expertise or patients choice.
Color Doppler ultrasound (CDU) has been proven to be a reliable AVF screening tool6, giving insight into preoperative morphological and functional characteristics of the vessels used for AVF construction7 but also having high sensitivity and specificity for diagnosing and characterizing JAS8-10.
Our approach was based on the fact that pre-operative CDU assessment of JAS allows an access centered approach by choosing the best therapeutic option in each fistula based on specific CDU criteria. The aim of this study was to evaluate immediate and long-term results of endovascular versus open surgical intervention of juxta-anastomotic venous stenosis of forearm radiocephalic fistulas using CDU based criteria for allocation to surgical or endovascular procedure.
METHODS
Study design, setting and patient population
A total of 1300 vascular access (VA) consultations belonging to 6 hemodialysis (HD) centers performed between 1 March 2011 and 31 October 2015 were retrospectively reviewed. Sixty-three patients with radiocephalic fistulas with hemodynamic significant JAS were identified. Patients were referred to VA consultations whenever impaired VA was suspected, including changes on physical examination, decreasing flow (Qa) measured by BTM® (Fresenius Medical Care), low Kt/v or cannulation difficulty. Following the routine procedure of our center, JAS from radiocephalic AVF that required intervention were selected and PTA or surgical procedures were performed in the interventional radiology unit as an outpatient procedure, after written informed consent. Follow-up examinations were performed systematically every month with clinical examination and Qa measurements.
Preoperative vascular evaluation
The diagnosis of JAS was made combining clinical data and CDU examination using a 7-15 MHz linear 38mm transducer (Ultrasound System; General Electric Pro scanner) carried out by the same experienced nephrologist.
VA assessment included combined physical examination and repeated CDU calculation of fistula flow rates while in the VA consultation. All dysfunctional AVF were evaluated with CDU allowing localization and characterization of the stenosis before allocating patients to surgical or PTA treatment.
Stenoses were considered hemodynamically significant in the presence of clinical signs associated with decreased dialysis dose, cannulation difficulties and low blood flow (<500 ml/min) or a blood flow reduction of more than 25% if Qa< 1000 ml/m. Otherwise Stenoses were considered hemodynamically significant only if there was at least one of the following additional ultrasound criteria:1 resistance index in the radial artery >0.7,2 residual stenosis diameter <2.0 mm.
Outcomes and study definitions
JAS was defined as the stenosis affecting the anastomosis site, including the radial artery and cephalic vein usually within 3 cm of the anastomosis, or a combination of both, sparing the puncture areas11.
The primary outcomes were post intervention primary patency (PP) and post intervention assisted primary patency (APP).
Post intervention primary patency (PP). PP is the interval from the time of any surgical or endovascular intervention until thrombosis or the time of measurement of patency12.
Post intervention assisted primary patency (APP). APP is the interval from the time of intervention until first access thrombosis or the time of patency measurement, including all intervening similar manipulations (surgical or endovascular interventions) designed to maintain the functionality of the access12.
Patients who were transferred to different angiographic centers, who received kidney allograft or died, were included until last known follow-up consultation with patent AVF.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation or median and interquartile range (IQR), and categorical variables as a percentage of the number of studied cases. Chi-square test was used to assess differences between proportions of categorical variables. Patency was analyzed using Life Tables and Kaplan-Meier statistics and the difference between surgical or endovascular interventions were compared using the log-rank test. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) 23.0 for windows (SPSS Inc. Chicago, Il, USA). P-values less than 0.05 were considered statistically significant.
RESULTS
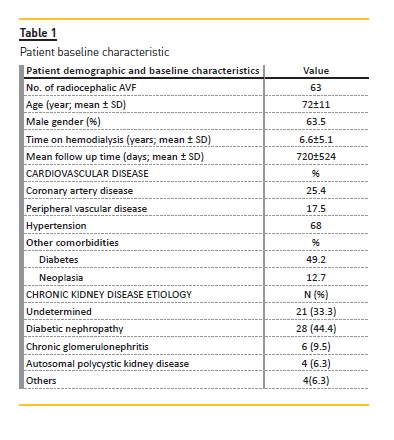
This study included 63 patients with a mean age of 72 ± 11 years, all undergoing hemodialysis for a mean of 6.6 ± 5.1 years. The majority of patients were male (63.5%; N=40) with 49.2% being diabetic (N=31) and 68% (N=43) hypertensive. Most patients had previous cardiovascular disease with 17.5% (N=11) having peripheral arterial disease and 25.4% (N=16) coronary heart disease. The most frequent etiology of patients chronic kidney disease was diabetic nephropathy, followed by the undetermined one (Table 1). The majority of patient referral was due to clinical diagnosis of JAS (N=24), followed by thrombosis (N=17), decreased Qa (N=10), decreased Qb (n=5), decreased Kt/v (N=3), outflow stenosis (N=2), cannulation difficulties (N=1) and one patient simply needed vascular access monitoring. After clinical and echo-Doppler assessment, A presumed diagnosis of JAS at the following sites was made: anastomosis site (n=3), radial artery (n=9) and radiocephalic vein (n=51). Four patients had simultaneous outflow stenosis.
Depending on CDU assessment of the AVF, patients were allocated into the two different treatment groups: Endovascular procedure was conducted when: 1) radial artery diameter was <2.5 mm; 2) significant calcified radial artery walls that could turn a proximal neo-anastomosis technically difficult; 3) new anastomosis with humeral artery, carrying out a higher risk of inducing AVF related ischemia like small caliber and significant calcified or stenotic radial or ulnar artery; 4) surgery would be required or there was a prediction of significant loss of vein length; 5) there was a damaged outflow vein wall with fibrosis proximal to the stenosis that preclude surgical revision; 6) multiple stenosis; 7) whenever the use of a central venous catheter would be necessary if the surgical revision was performed.
The surgical approach with the creation of a proximal neo-anastomosis was preferentially conducted whenever: 1) the diameter of radial artery was >3mm; 2) there was an intact cephalic vein wall with a diameter >3mm without any nearby stenosis allowing wider preservation of the vein length and easier cannulation the day after the procedure; 3) when there were more than 2 PTA in less than 3 months.
After applying CDU criteria, surgical revision was proposed and performed in 43 cases, with creation of a new proximal fistula the most common procedure (N=41), and 2 patients having surgical angioplasty performed. Of the 41 patients submitted to a new anastomosis, 2 patients had immediate thrombosis after procedure, needing construction of a new humeral-cephalic AVF and at the same time a HD central venous catheter. In 17 patients JAS was diagnosed after thrombosis had already occurred. In all cases, the outflow vein was patent, allowing its characterization.
In these patients, 16 were successfully treated surgically with CDU guiding the best location for the new proximal anastomosis. Only one patient needed a central venous catheter. One patient had multiple stenoses that were solved with a hybrid approach.
Patients with occlusion of the outflow vein, needing thrombectomy, were excluded from the study. The angiographic procedure was performed in 20 patients; all patients had functioning AVF after the procedure.
There was no misdiagnosed stenosis with CDU compared with angiography in the endovascular group and surgical patients had no significant outflow stenosis after JAS correction.
Mean follow-up time was 720 ± 524 days (median: 706, IQR: 214-1204) with a maximum follow-up of 4.6 years. During the follow-up, 10 patients died, 3 were transplanted and 2 were transferred to another angiographic center.
Thrombosis was observed in 3 AVF, 2 from the endovascular group and 1 from the surgical group. Thirty-five per cent of patients (7/20) submitted to a first angiographic proceeding relapsed, not statistically different from the 18.6% (8/43) who relapsed when submitted to initial surgical approach (p=0.155).
In the surgical group, primary patency was 92% and 84% at 6 and 12 months respectively, significantly better than the endovascular group (76% and 47%: p=0.013). There was no significant difference in the assisted primary patency between the interventional groups at 12 months: 94 % in the endovascular vs. 93% in the surgical group (p= 0.542). (Figure 1; Table 2).
DISCUSSION
The cornerstone of AVF salvaging is the accurate identification of stenotic lesions. In radiocephalic AVF, JAS is the most common finding, even more common after CDU assessment, as previously reported in other studies13,14. There is still controversy about which method is the best approach to manage JAS, surgery or endovascular proceeding. Recent reports suggest that stenosis can be safe and successfully managed using either endovascular procedure or surgery13,15.
Previous studies show that surgical and endovascular interventions do not differ significantly when it comes to primary patency although re-intervention rates may differ8,16,17. Although PTA is less invasive, there is a higher restenosis rate5,16,18 probably due to ballooninduced vascular wall injury which accelerated the neointimal hyperplasia promoting more neo-intimal hyperplasia and stimulating smooth muscle cell proliferation19.
Restenosis develops faster than a virgin stenosis. The key is in appropriate timing of PTA procedures20. In our study, there were no significant differences between primary assisted patency but primary patency was significantly better in surgical patients even when we included patients whose first presentation of JAS was thrombosis, a sign of worse prognosis in access survival. Similar to our study, PP at 18 months was higher in the surgically repaired group as demonstrated in the meta-analysis of four nonrandomized cohort studies15. This was noteworthy with post-treatment primary and APP rates after proximal neo-anastomosis21. A small study that included only endovascular treatment of JAS demonstrated that longterm good results are possible, except when the residual stenosis after the first procedure is 50% or more22.
Patients should know that when submitted to endovascular proceedings, multiple maintenance interventions might be needed5,23.
Independently of the procedure, either surgical or endovascular, the final decision usually depends on individual preference, technique availability, surgeon, interventional radiologist and nephrologist experience, as well as economic and material resources. Our perspective is that the two methods are complementary and not competitive and in JAS treatment CDU could be the answer to which is the best treatment for each access. CDU is a non-invasive method that requires no radiation or contrast. It allows stenosis characterization and hemodynamic evaluation, establishing any need for intervention in the AVF stenosis. The CDU criterion to allocate patients into 2 groups of intervention proposed in this study allowed an individualized vascularaccess-centered approach. A better patient allocation significantly decreases the number of re-interventions, having a positive impact on PP, patient morbidity and costs by also decreasing major complications and inconvenience associated with the intervention.
Our therapeutic option took into consideration the immediate risk of vascular access loss when a more complex surgery is performed, such as in AVF with a <2.5 mm diameter, significant calcified radial artery walls or damaged outflow vein wall with fibrosis or whenever there is an extensive loss of venous capital, especially when needing a central venous catheter to undergo hemodialysis. Even with this caution, 2 patients had immediate thrombosis after surgical procedure.
PTA is not free of complications such as thrombosis and vein wall rupture with severe and uncontrolled hemorrhage needing immediate access ligation after balloon dilation. However, there is no proven risk factor associated with worse outcome regarding PTA stenosis treatment and the risk of immediate access loss seems to be lower compared to surgery. PTAs main benefit is still preservation of proximal vein length and higher immediate success after procedure. The surgical approach can be associated with higher primary failure and complications as insufficient vein length for cannulation after the procedure. In these cases, we propose an initial PTA assuming that several other endovascular proceedings may be needed.
Depending on the time of the recurrence, if inferior to 3 months, we propose the construction of a new proximal AVF, even though a temporary central venous catheter may be needed. PTA is preferred if there is a high ischemic risk related to a new AVF construction using the humeral artery.
Study limitations include its retrospective design, a single vascular access center and the lack of ability to detect significant predictors of access failure as a result of the small number of patients. Using the pre-operative CDU assessment, our team chose the salvage technique without randomization, which can be also a study limitation.
To the best of our knowledge, this is the first study that allocates JAS to endovascular or surgical therapeutic intervention based on CDU assessment. This approach with specific allocation criteria choosing the best option in each fistula allowed the correct diagnosis of the lesion, improved the global results of the treatment and optimized the financial resources by reserving PTA for selected cases where surgery could be more difficult with higher risk of access loss.
References
1. Suttie S. Geeta P, Henderson N, et al. Natural history of upper limb arterio-venous fistulae for chronic hemodialysis. J Vasc Access 2012;13(3):332-337. [ Links ]
2. National Kidney Foundation. K/DOQI clinical practice guidelines for vascular access, update 2002. Am J Kidney Dis 2001;37(1):S137-S181. [ Links ]
3. Turmel-Rodrigues L. Dilatation is usually the best treatment for stenosis of the arteriovenous hemodyalisis fistula. Nat Clin Pract Nephrol 2008;4(3):116-117. [ Links ]
4. Tordoir J. Early pre-emptive intervention might reduce AVF access loss. Nat Rev Nephrol 2014;10:9-10. [ Links ]
5. Tessitore N, Mansueto G, Lipari G, et al. Endovascular versus surgical preemptive repair of forearm arteriovenous fistula juxta-anastomotic stenosis: analysis of data collected prospectively from 1999 to 2004. Clin J Am Soc Nephrol 2006;1:448-454. [ Links ]
6. Tonelli M, James M, Wiebe N, et al. Ultrasound monitoring to detect access stenosis in hemodialysis patients: a systematic review. Am J Kidney Dis 2008;51:630-640. [ Links ]
7. Lomonte C, Meola M, Petrucci I, Casucci F, Basile C. The key role of color doppler ultrasound in the work-up of hemodialysis vascular access. Semin Dial 2015;28(2):211-215. [ Links ]
8. Napoli M, Prudenzano R, Russo F, Antonaci A, Aprile M, Buongiorno E. Juxta-anastomotic stenosis of native arteriovenous fistulas: surgical treatment versus percutaneous transluminal angioplasty. J Vasc Access 2010;11(4):346-351. [ Links ]
9. Koseoglu K, Akar H, Cildag B, Ozsunar Y, Gayret P. Resistive index measurement of native hemodialysis arteriovenous fistula feeding artery as a predictor for fistula dysfunction. ASAIO J 2004;50:577-580. [ Links ]
10. Tordoir J, Canaud B, Haage P, et al. EBPG on vascular access. Nephrol Dial Transplant 2007;22(2):ii88-ii117. [ Links ]
11. Oakes D, Sherck J, Cobb L. Surgical salvage of failed radiocephalic arteriovenous fistulae: techniques and results in 29 patients. Kidney Int 1998;53:480-487. [ Links ]
12. Sidawy A, Gray R, Besarab A, Henry M, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. Vasc Surg. 2002;35(3):603-610. [ Links ]
13. Asif A, Gadalean FN, Merrill D, et al. Inflow stenosis in arteriovenous fistulas and grafts: a multicenter, prospective study. Kidney Int 2005;67:1986-1992. [ Links ]
14. Beathard GA, Arnold P, Jackson J, Litchfield T. Aggressive treatment of early fistula failure. Physician Operators Forum of RMS Lifeline. Kidney Int 2003;64:1487-1494. [ Links ]
15. Argyriou C, Schoretsanitis N, Georgakarakos E, et al. Preemptive open surgical vs. endovascular repair for juxta-anastomotic stenoses of autogenous AV fistulae: a metaanalysis. J Vasc Access 2015;16(6):454-458. [ Links ]
16. Long B, Brichart N, Lermusiaux P, et al. Management of perianastomotic stenosis of direct wrist autogenous radialcephalic arteriovenous accesses for dialysis. J Vasc Surg 2011;53:108-114. [ Links ]
17. Tordoir J, Bode A, Peppelenbosch N, Van der Sande F, de Haan M. Surgical or endovascular repair of thrombosed dialysis vascular access: Is there any evidence? J Vasc Surg 2009;50:953-956. [ Links ]
18. Lu F, Liu L, Lu Z. One-year patency rate of native arteriovenous fistulas reconstructed by vascular stripping in hemodialysis patients with venous neointimal hyperplasia. J Vasc Surg 2015;61:192-196. [ Links ]
19. Chang C, Ko P, Hsu L, et al. Highly increased cell proliferation activity in the restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: implication in prevention of restenosis; Am J Kidney Dis. 2004 Jan;43(1):74-84. [ Links ]
20. KudlickaJ, Kavan J, Tuka V, Malik J. More precise diagnosis of access stenosis: ultrasonography versus angiography. J Vasc Access 2012;13(3):310-314. [ Links ]
21. Kwon H, Choi J, Ko H, et al. Comparison of surgical and endovascular salvage procedures for juxta-anastomotic stenosis in autogenous wrist radiocephalic arteriovenous fistula. Ann Vasc Surg 2014;28:1840-1846. [ Links ]
22. Mortamais J, Papillard M, Girouin N, et al. Endovascular treatment of juxta-anastomotic venous stenoses of forearm radiocephalic fistulas: long-term results and prognostic factors. J Vasc Interv Radiol 2013;24:558-564. [ Links ]
23. Asif A, Lenz O, Merrill D, et al. Percutaneous management of perianastomotic stenosis in arteriovenous fistulae: results of a prospective study. Kidney Int 2006;69:1904-1909. [ Links ]
Ana Pimentel
Nephrology Department, Centro Hospitalar do Algarve
Faro, Portugal
E-mail: anappimentel@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: 13 Jan 2018
Accepted in revised form: 1 Mar, 2018














