Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.2 Lisboa jun. 2018
ORIGINAL ARTICLE
Evaluation of the Portuguese kidney transplant allocation system: comparative results from a simulation
Bruno A Lima1, Helena Alves2
1 Oficina de Bioestatística, Vilar Formoso, Portugal
2 Instituto Nacional de Saúde, Dr. Ricardo Jorge, Porto, Portugal
ABSTRACT
The distribution of such a scarce resource as deceased donor kidneys should be made by observing a balance between fairness, efficiency and flexibility. Before implementing a new kidney allocation system, these principles should be evaluated and assured objectively.
In this article we compare the renal transplant donor-recipient pair selection system implemented in Portugal in 2007 with the Eurotransplant (ET) and United Kingdom (UK) systems.
We simulated data for 500 waitlist kidney transplant candidates and 70 deceased donors. Each of the 70 donors was allocated to the best pair of listed candidates, taking into account the criteria of the three allocation systems under analysis. Subsequently, we compare the selected candidates groups to kidney transplant.
The Portuguese organ allocation model selects candidates with a greater number of incompatibilities with the donor compared to the other two models. Under the Portuguese systems rules, candidates have a greater age difference with the respective donors (median = 12.5 years) than those selected by the ET system (10 years) or the UK system (8 years). The Portuguese model selected more hypersensitized candidates (15%), but this difference was not statistically significant when compared to the percentage of hypersensitized patients selected by the ET model (10.7%).
The Portuguese model has less equity than the other two models under analysis, since the observed disadvantages regarding the number of incompatibilities and age differences with the respective donor are not compensated for by the selection of patients with longer time on dialysis.
Key-words: access to transplantation, deceased donor, kidney transplant, organ allocation
INTRODUCTION
When possible, renal transplantation is the best renal replacement therapy available for end stage renal disease patients. Transplantation is associated with a lower risk of death than is dialysis1.
In 2009, Portugal registered the highest number of performed kidney transplants in its history both in absolute terms2 and in relative terms compared with the remaining countries in the European Union (EU)3.
In that year, Portugal reached first position within 28 EU members ranking of kidney transplants per million inhabitants. Afterwards and until 2014, Portugal consecutively fell in this ranking, but 2015 saw a reversal of this trend,3 one we hope will continue in forthcoming years.
A kidney allocation system, in addition to being transparent, must seek a balance between fairness, efficiency and flexibility in the distribution of these organs. The efficiency criterion allocates kidneys looking for the greatest good for the greatest number of patients; using a fairness criterion kidneys would be allocated to those patients who need them the most4.
In other words, the candidates chosen as possible donors should be those with the higher chance of transplant success, in addition to being those who have waited longer for a graft5. Equitable patient treatment means to treat differently what is different; hence, equity in access to kidney transplantation lies in the balance between fairness and efficiency in organ distribution.
AIM
The aim of this work is to evaluate and compare three models for deceased donor kidney allocation.
The first model is adapted directly from the scoring criteria of the Portuguese regulations in ordinance n.º 6537/2007; the second based on the criteria used by the ET system6 and the third adapted from the United Kingdom kidney allocation system7.
METHODS
In this analysis, we generated data to simulate a waiting list of 500 renal transplant candidates. For each of these simulated patients we assigned an age, time on dialysis, blood group, human leukocyte antigen (HLA) system typing for A*, B* and DRB1* loci, and anti-HLA antibodies to impute the respective Panel Reactive Antibody calculated value (cPRA)8. We generated these data as described elsewhere9. Briefly, we assigned transplant candidates typing, taking into account published HLA allelic and haplotype frequencies10. We choose blood groups so that 43% were from group A, 3% from group AB, 8% from group B and the rest from group 0. Further, we assigned Anti-HLA antibodies so that 4.6% had a cPRA ≥ 85%; 8.6% a cPRA value between 50% and 85%; 6.8% a cPRA between 0% and 50% and the remaining 80% of the candidates a cPRA equal to 0%. With these data, we classified candidates with cPRA values higher than 85% as belonging to the Acceptable Mismatch (AM) Program.
Data were also generated for 70 simulated deceased donors9 to be distributed by the best candidates on the waiting list according to kidney allocation rules used in Portugal, the ET and United Kingdom. Briefly, we generated donors ages from a normal distribution with mean 55 and standard deviation 15; blood groups of each donor were randomly assigned, taking into account distributions published from Portuguese blood donors11 and we generated the respective HLA typing randomly from published allelic and haplotype frequencies10 of voluntary bone marrow donors12.
For the three models and for each donor, we selected only those candidates without donor specific antibodies (negative virtual crossmatch) and AB0 blood group compatible. The methods used for candidates selection through the first model, Portuguese (PT) kidney allocation system (KAS) ( Table I), are described in detail elsewhere9.
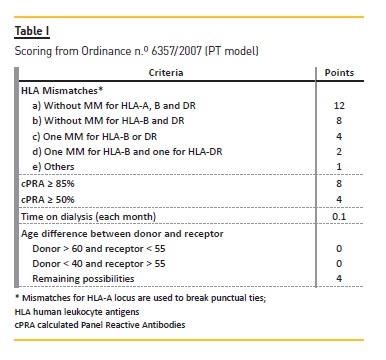
For the second model, ET KAS (Figure 1), we selected only candidates within the Eurotransplant Senior (ES) program (i.e., older than 65 years), with a negative virtual crossmatch (vXM) and AB0 identical. From these we selected the two candidates with longer time on dialysis. When the donor was less than 65 years old, selected candidates were those within the AM program with a negative vXM and AB0 compatible with at least 0 mismatches for locus DRB1* (MM-DRB1) or 1 MMDRB1 and 0/1 mismatches for locus B* (MM-B). Only if there were no AM program candidates available did we select from the remaining candidates those AB0 identical. Afterwards we scored candidates according to the ET-KAS (Table II) – we calculated mismatch probabilities (MMP) taking into consideration HLA allelic frequencies from Portuguese bone marrow donors 10 and AB0 blood group type frequencies from Portuguese blood donors11. We marked the two candidates with higher score as transplanted. This process was repeated with the remaining donors until we reached a pool of 140 transplanted patients.
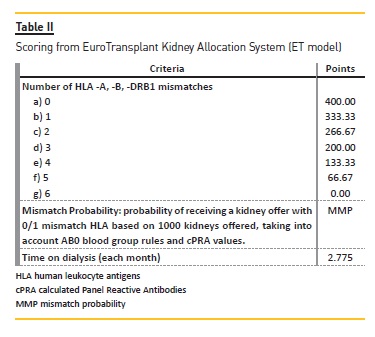
With the same 500 waitlist candidates and the same 70 donors, we applied the United Kingdom kidney allocation system (UK-KAS). In this third model, for the first donor we selected candidates with a negative vXM and AB0 identical (with the exception of AB candidates who can receive from A donors and B candidates who can receive from 0 donors). We prioritized these patients over candidates with 000 mismatches for HLA-A*, -B*, DRB1* (MM-HLA) and cPRA ≥85% or homozygotes for DRB1* (Figure 2), followed by candidates with 000 MMHLA, followed by the remaining candidates according to the score assigned by UK-KAS (Table III). Using this order, we marked the first two candidates as transplanted.
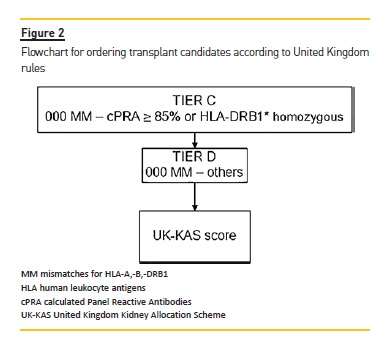
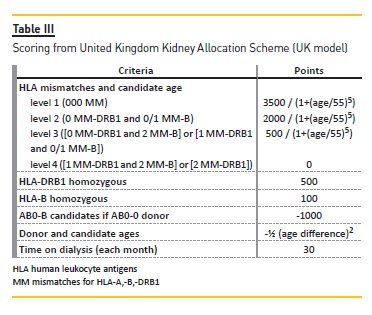
We repeated this process with the next donor and the remaining candidates until we selected 140 candidates as transplanted.
Characteristics of candidates selected as transplanted within each one of the models were compared.
Median time on dialysis, candidates ages and differences between donor and recipient were compared using the Kruskal-Wallis test. The chi-square test (or Fisher exact test when appropriate) was used to compare recipients rates for the cPRA groups and HLA mismatches groups for each model. P-values < 0.05 were considered statistically significant. We performed all statistical analysis and graphic representation with RStudio software for R programming language.
RESULTS
We found no statistically significant differences in the ages of selected candidates in each of the three models under analysis (Table IV). That said, when we calculated age differences between donor and receptor, we verified that the Portuguese (PT) model selected candidates with higher age differences. Median age difference between donor and recipient was 12.5 years in the PT model, while the UK model selected candidates with a median age difference of only 8 years (p <0.001) and for ET allocation model median age difference was 10 years (p = 0.03).
The ET model selected candidates with a shorter time on dialysis (62.5 months); this result was not statistically different from median time on dialysis observed for the PT models recipients (67 months). There are no differences to highlight regarding blood group distributions of selected candidates in each model.
Concerning the HLA mismatches between the selected recipients and their donors, the PT model was the one that selected candidates with more HLA mismatches when compared to the other models. In the PT model only 60 (42.9%) recipients had up to 3 MM-HLA, but the ET model selected 102 (72.8%, p <0.001) and in the UK model, 83 (59.3%, p = 0.01) recipients had at most 3 MM-HLA. Furthermore, if we take into consideration only MM for loci B * and DRB1 *, the PT model selected more recipients with 0 MM-BDR (7, corresponding to 5%). On the other hand, the PT model selected 64 (45.7%) recipients with 3 or 4 MM-BDR while the ET model selected only 34 (24.3%, p <0.001) and only 36 (25.7%, p <0.001) recipients were selected with the UK model.
The PT model was the one that selected a higher number of hypersensitized candidates 21 (15%); the ET model selected 15 (10.7%; there were no statistically significant differences with the PT model) and the UK model selected only 4 (2.9%, p <0.001) hypersensitized candidates.
DISCUSSION
The Portuguese KAS chooses waitlist candidates with more MM-HLA with the donor than the ET and UK KAS does. The number of MM-HLA is associated with an increased risk of poorer transplant outcomes13. Additionally, de novo post transplant donor specific anti HLA antibodies due to HLA mismatches at transplantation are associated to graft failure14. These disadvantages for Portuguese transplant candidates are not balanced by the selection of candidates with higher time on dialysis when compared with the other two models in this analysis (differences statistically not significant).
Prior to the implementation of ordinance nº 6537/2007, in Portugal, deceased donor kidney allocation was primarily made taking into account the number of HLA compatibilities with the donor. After 2007, transplanted patients were mainly those with a longer time on dialysis15 regardless of the number of HLA compatibilities with the donor. As a matter of fact, the Portuguese model does not penalize enough transplant candidates with several MM-HLA with their potential donors. For instance, a transplant candidate with 6 MM-HLA has one point on the PT KAS; the same point attributed to a candidate with only 2 MM-DRB1* or with only 2 MM-B*, and just one point less than a candidate with 1 MM-B* and 1 MM-DRB1*. In the current immunosuppression era, MM-DRB1* have been described as the more relevant HLA mismatches associated with post-transplant rejection episodes16. While the UK model gives a great weight to MM-DRB1* in its scoring criteria, the ET model gives equal weight to the MM for HLA-A *, -B * and -DRB1 * loci.
If one of the main goals in the implementation of the current PT model was to mitigate the hypersensitized candidates waiting time disadvantage17, we verified that the PT model selected the highest percentage of hypersensitized patients (15%) compared to the other two models; though the difference between this and the ET model is not statistically significant. The Eurotransplant KAS applies the AM program which prioritizes hypersensitized candidates; yet the PT KAS gives extra points to hypersensitized (8 points) and sensitized (4 points) candidates. With these extra points, the PT model only selects 58.6% of candidates with a cPRA <50%, though by the ET model they are 82.1% and by the UK model 87.9%.
In this simulation, for each waitlist candidate, we calculated a cPRA, when in fact, under ordinance n.º 6537/2007, Portuguese candidates on the waiting list are classified (or not) as sensitized patients, taking into account only results from the Panel Reactive Antibody by the Complement Dependent Cytotoxicity (PRA-CDC) screening method. PRA-CDC results are not an accurate gauge of the likelihood of finding an incompatible donor18 and are used to underestimate the real number of hypersensitized candidates on the waiting list19. We calculate PRA-CDC as a quotient of the number of individuals whose lymphocytes had a positive cytotoxic reaction with a patient serum by the total number of tested individuals. Since these groups of tested individuals may not be representative of potential donors HLA profiles and given the lack of cytotoxic reactions sensitivity, PRA-CDC has been replaced by cPRA as a measure of allosensitization for waitlist transplant candidates20,21. Inevitably, in Portugal, as in the ET and UK models, cPRA values will also be implemented as a criterion in the distribution of kidneys from deceased donors. When applied, cPRA will increase the number of hypersensitized candidates on the waiting list, and if applied to the current PT KAS, will dilute the extra score few patients currently benefit from.
Although there were no statistically significant differences for candidates ages selected by the models, recipients on the PT model were those with greater age difference between patients and their respective donors. The Eurotransplant KAS has the ES program in which donors over 65 are primarily targeted to candidates over 65; the UK KAS assigns penalty points directly proportional to the age difference between candidate and potential donor, whereas the PT KAS only penalizes more extreme cases of age differences between candidates and donors, which we verified in the results obtained.
Moreover, as the population is aging, in Portugal, we expect that more kidneys from elderly people will become available in the future. In an equitable kidney allocation model, older donors should be targeted primarily at older candidates, so measures similar to those of the ES program should be considered instead of the current point system used in the PT model. Regardless, the ES program does not take into account the MM-HLA as a way to reduce the organ ischemia time inherent in the distances between the Eurotransplant member countries. In Portugal, given geographical dimensions, MM-HLA should not be overlooked since the reduction in the number of MM-HLA is a factor that can counterbalance the disadvantages associated with an older organ22.
The definition of clear and equitable rules for deceased donors organ allocation can also be used in a better distribution of kidneys from living donors. As an illustration, see the example of a living donor exchange program23 where an incompatible donor-receptor pair can be exchanged with another two or three incompatibles pairs. In these programs, the first criterion for choosing the pairings is to maximize the number of possible transplants, but if there are still several possibilities of choice, then the criteria applied to allocation of deceased donors can be used to resolve those ties24.
LIMITATIONS
Above all, we must emphasize that we obtained the results presented here from simulated data and not from real waitlist transplant candidates. The lack of open data on renal transplant candidates and on organ donors25 does not allow us to make a deeper analysis in the application of these kidney allocation models.
For instance, we couldnt analyze how the number of children, the number of candidates for re-transplantation or multi-organ waitlist candidates can condition patients selection for a given group of donors. Also, the fact that transplant candidates can register on two transplant waiting lists (something sui generis to Portuguese candidates26) is not part of our analysis.
Application of deceased donor kidney distribution rules depends on detailed knowledge of waitlist transplant candidates and the evolution of these donors characteristics.
To this end, it is necessary to define systematic and objective measures27 that are clinically useful and can be scrutinized by all interested parts. Accordingly, the PT model, due to its lack of simplicity, transparency and equity9, needs to be discussed and evaluated so that it can be corrected or at least improved. Here, we seek to compare the PT model with two other solidly implemented models (the ET model and the UK model), identifying possible ways to upgrade the former.
More than 10 years after ordinance 6357/2007 was implemented and without relevant changes in this period, surely the current state of the art allows us to find points we need to correct. Furthermore, we must base needed corrections or changes on objective data and scientifically on verifiable proven evidences. The availability of open data regarding renal transplantation activity 25 is a sine qua non for achieving this goal.
CONCLUSION
In conclusion, we want to emphasize three main ideas. First, the current Portuguese KAS can and should be replaced by a more equitable allocation system; second, a new KAS with objective and more transparent criteria can and must be defined and discussed; and third, those with responsibilities for defining and implementing a new KAS, in Portugal, do not need to reinvent the wheel. At this point, we should be able to process data, compare it with other experiences, looking abroad for allocation systems that work and can help us to improve and fulfill our purposes.
References
1. Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11(10):2093-2109. [ Links ]
2. Lima BA, Alves H. Evolução da atividade de transplantação renal em Portugal: dados públicos de 2003 a 2015. Obs – Bol Epidemiológico 2017; 6(18):24-27. [ Links ]
3. Lima BA, Alves H. Atividade do transplante renal de 2003 a 2016: Portugal na União Europeia a 28. Obs – Bol Epidemiológico 2017; 6(19):16-19. [ Links ]
4. Norman DJ. The kidney transplant wait-list: allocation of patients to a limited supply of organs. Semin Dial 2005; 18(6):456-459. [ Links ]
5. Lima BA, Mendes M, Alves H. Kidney transplant allocation in Portugal. Port J Nephrol Hypert 2013; 27(4):313-316. [ Links ]
6. Eurotransplant Manual –version 5.5. EuroTransplant Foundation. Available at http://www.eurotransplant.org/cms/index.php?page=et_manual. Accessed January 2nd, 2018. [ Links ]
7. Kidney Transplantation: Deceased Donor Organ Allocation. NHS Blood and Transplantation. Available at https://www.odt.nhs.uk/transplantation/tools-policies-and-guidance/policies-and-guidance/. Accessed January 2nd, 2018. [ Links ]
8. Cecka JM. Calculated PRA (CPRA): The new measure of sensitization for transplant candidates: special feature. Am J Transplant 2010; 10(1):26-29. [ Links ]
9. Lima BA, Alves H. Selection of donor-recipient pairs in renal transplantation: comparative simulation results. Acta Med Port 2017; 30(12):854-860. [ Links ]
10. Lima BA, Alves H. HLA-A, -C, -B, AND -DRB1 allelic and haplotypic diversity in bone marrow volunteer donors from northern Portugal. Cells Tissues Organs; 2013; 16(1):19-26. [ Links ]
11. Duran J, Chabert T, Rodrigues F, Pestana D. Distribuição dos grupos sanguíneos na população portuguesa. AB0 2007; 29:5-17. [ Links ]
12. Lima BA. Bone marrow volunteer donors recruitment in northern Portugal. Acta Med Port 2011; 24:301-306. [ Links ]
13. Lim WH, Chadban SJ, Clayton P, et al. Human leukocyte antigen mismatches associated with increased risk of rejection, graft failure, and death independent of initial immunosuppression in renal transplant recipients. Clin Transplant 2012; 26:428-437. [ Links ]
14. Castro A, Malheiro J, Tafulo S, et al. Role of de novo donor-specific anti-HLA antibodies in kidney graft failure: a case-control study. HLA 2017; 90(5):267-275. [ Links ]
15. Lima BA, Mendes M, Alves H. Kidney transplantation in the north of Portugal: donor type and recipient time on dialysis. Port J Nephrol Hypert 2013; 27(1):23-30. [ Links ]
16. Broeders N, Racapé J, Hamade A, et al. A new HLA allocation procedure of kidneys from deceased donors in the current era of immunosuppression. Transplant Proc 2015; 47(2):267-274. [ Links ]
17. Fonseca NM, Nolasco F. Kidney allocation: new contributions to an ongoing challenge. Acta Med Port 2017; 30(12):833-834. [ Links ]
18. Zachary A, Braun W. Calculation of a predictive value for transplantation. Transplantation 1984; 39(3):316-318. [ Links ]
19. Lima BA, Mendes M, Alves H. Hypersensitized candidates to kidney transplantation in Portugal. Port J Nephrol Hypert 2013; 27(2):77-81. [ Links ]
20. Magriço R, Malheiro J, Tafulo S, et al. Implications for patients waiting for a kidney transplant of using the calculated panel reactive antibody (cPRA). Port J Nephrol Hypert 2016; 30(2):185-193. [ Links ]
21. Malheiro J, Tafulo S. Clinical implications of anti-HLA antibodies testing in kidney transplantation. Port J Nephrol Hypert 2018; 32(1):5-14. [ Links ]
22. Foster BJ, Dahhou M, Zhang X, Platt RW, Hanley JA. Relative importance of HLA mismatch and donor age to graft survival in young kidney transplant recipients. Transplantation 2013; 96(5):469-475. [ Links ]
23. Lima BA, Alves H. A Portuguese living donor exchange programme. Cells Tissues Organs; 2012; 15(2):123-129. [ Links ]
24. Lima BA, Alves H. The Portuguese match algorithm in the kidney paired donation program. Cells Tissues Organs; 2010; 13:25-32. [ Links ]
25. Lima BA. A call for open data of renal transplantation in Portugal. Port J Nephrol Hypert 2017; 31(3):155-157. [ Links ]
26. Lima BA. Acesso ao transplante de rim de dador cadáver no norte de Portugal. Dissertação de Mestrado em Saúde Pública. Faculdade de Medicina da Universidade do Porto. 2011. Available at http://hdl.handle.net/10216/22118. Accessed December 2nd, 2017 [ Links ]
27. Lima BA, Mendes M, Alves H. Measuring kidney transplantation activity. Port J Nephrol Hypert 2014; 28(2):171-176. [ Links ]
Bruno A Lima Oficina de Bioestatística
Rua do Comércio, 42, 6355-248 Vilar Formoso
E-mail: balima78@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: Feb 16, 2018
Accepted in revised form: May 21, 2018














