Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.2 Lisboa jun. 2018
REVIEW ARTICLE
Supportive care in advanced chronic kidney disease: Comprehensive conservative care
C Belino1, C Meng2, R Neto2,3, E Gonçalves4, M Pestana2,5
1 Department of Nephrology, Centro Hospitalar de Vila Nova de Gaia e Espinho, Vila Nova de Gaia, Portugal
2 Department of Nephrology, Hospital de São João, Porto, Portugal
3 Department of Nephrology, Pre-dialysis Consult, Hospital de São João, Porto, Portugal
4 Department of Pallitave Care, Hospital de São João, Porto, Portugal
5 Ethics Committee of Hospital de São João, Porto, Portugal
ABSTRACT
Incident and prevalent patients on dialysis are progressively older, with high comorbidity burden and functional dependency. Many could have benefited from a conservative approach, since considerations of symptoms, autonomy, quality of life and hospital-free days are sometimes more important for patients and families than survival. As result, nephrologists around the world are facing challenges to determine which treatment best fits their patients. Comprehensive conservative care in chronic kidney disease care has been recently defined as a holistic, multidisciplinary and patient-centered approach for care of patients with stage 5 CKD. It does not include dialysis, and a shared-decision-making process and advanced care planning are central pillars, providing a way to meet patient and families goals. This review will focus on comprehensive conservative care in CKD in order to provide a communication framework for decision-making process as a guide for nephrologists and other health care professionals.
Keywords: Advanced kidney disease, comprehensive conservative care, palliative care
INTRODUCTION
Chronic kidney Disease (CKD) is an increasing global health problem and is related to the ageing of the population1-6. The majority of incident and prevalent dialysis patients are elderly (age ≥ 65 years), most of them with significant comorbidities and/or reduced functional capacities. Dialysis may impose an additional symptom burden without bringing improvements in survival or quality of life and with greater health costs7-10. Considering this, nephrologists around the world are facing a dilemma to determine which patients are more suitable for conservative treatment as an alternative to dialysis and how to implement and manage it. To assist with that decision, this review will focus and discuss the aspects of comprehensive conservative care in ESRD.
THE BURDEN OF CHRONIC KIDNEY DISEASE
Advances in health care have contributed to a prolonged life span, with a major increase in chronic medical conditions such as cardiovascular and metabolic diseases. Worldwide, about 60% of deaths are related to chronic conditions and this number is expected to rise by 15% by 20201. CKD already affects about 15% of the world population2 and has resulted in almost one million deaths worldwide, signifying a rise of 134% between 1990 and 20133. Also, CKD is the 15th and 20th cause of years lived with disability4 and disabilityadjusted life years5. In Portugal, CKD is responsible for 29% of the annual mortality rate (per 100,000 people), with a rise of 84.8% since 1990. It is also responsible for 782.2 annual years of healthy life lost (per 100,000 people) which have increased by 23.9% since 19906.
The costs are extremely high7. These results are expected to grow further with the ageing of the population.
In fact, older patients are the fastest-growing group of incident ESRD patients and have nearly doubled since 19978. Presently, more than half of patients initiating dialysis are > 60 years of age9.
THE CHALLENGE OF ADVANCED
KIDNEY DISEASE CARE
Recent evidence suggests that the overall benefit of dialysis is not the same for all patients8,9. For a certain type of patient (advanced age, presence of severe comorbidities or/and poor functional status) the benefit of dialysis in survival is residual and is acquired with a greater time spent in hospital services (including scheduled dialysis treatments), with adverse consequences on quality of life and a greater social burden10-16. Those who were managed without dialysis maintained their functional status for longer and with better life satisfaction scores17. Additionally, older patients with slower rates of CKD progression can survive a significant amount of time without dialysis10,13.
For patients who are already on dialysis, withholding treatment in those with very poor prognosis or in whom dialysis may aggravate clinical condition can be a reasonable option and medical treatment can provide good results18. Also, global social and economic crisis have created the need for adequate and just allocation of resources19. Portugal has one of the highest rates of incident (226.5 new cases per million) and prevalent (1661.9 cases per million) ESRD patients in Europe and 2.5% of health care costs are attributable to hemodialysis treatments19. Considering all this, identifying the ideal patient for conservative treatment and how to approach and manage the care are issues of great debate and priority in nowadays.
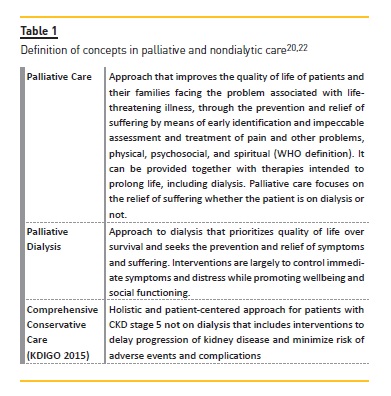
DEFINING CONCEPTS
A range of terms have been used in relation to nondialytic care in ESRD but without a clear definition. In recent years, there has been an effort to define and organize distinctive concepts (Table I)20-22. The KDIGO 2015 conference on supportive care introduced the concept of comprehensive conservative care in the context of CKD stage 5 not on dialysis, including the interventions to delay progression of kidney disease and minimize risk of adverse events or complications and the palliative care principles and philosophy applied, instead of renal palliative care because many health care professionals and patients consider palliative care and terminal care as synonymous, which is not correct20,22.
COMPREHENSIVE CONSERVATIVE CARE
Comprehensive conservative care for CKD was proposed as a term able to reflect the full extent of conservative management20. Three distinct groups were distinguished:
1. Those where conservative care is either chosen or medically advised.
2. Those receiving choice-restricted conservative care where resource constraints have prevented or limited access to renal replacement therapy.
3. Those with unrecognized stage 5 CKD, where CKD is present but has not yet been recognized or diagnosed.
Differences in patterns of these groups are seen between lower-middle-income countries, where access C Belino, C Meng, R Neto, E Gonçalves, M Pestana to dialysis may be limited, and high-income countries, where dialysis is more widely available20. Further, we will discuss the components of comprehensive conservative care.
Interventions to delay progression of kidney disease and minimize risk of adverse events and complications
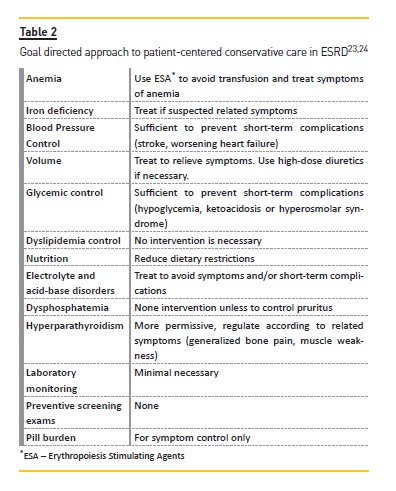
This point regards the management of CKD-related issues according to actual guidelines, including anemia, blood pressure, volume, metabolic disorders, electrolyte and acid-base disorders and CKD-mineral and bone disorders. In Table 2, there are examples of an individualized and patient-centered approach to care for patients with ESRD23,24.
The approach is goal direct and prioritizes symptomatic control and avoidance of potential complications that can be manageable in a non-hospital environment.
Minimizing the use of drugs and invasive interventions is fundamental. Erythropoiesis-stimulating agents and iron therapy can help to improve weakness, fatigue and dyspnea. Blood pressure control should consider recommended goals but must be adapted to patient age and comorbidities, with attention to deleterious side effects, such as orthostatic hypotension and cognitive decline, particularly in those patients with longstanding hypertension and vascular disease. There is no evidence of benefit of one class of drugs over another in patients who choose conservative care. Volume management helps to ameliorate fatigue and dyspnea, increases quality of life and reduces hospital visits. Limitation of dietary restrictions can improve some nutritional parameters, reduce the risk of hypoglycemia and may contribute to better quality of life. Phosphorus-biding agents, vitamin D analogues and/or calcimimetics to control hyperparathyroidism can reduce pruritus, bone pain and muscle weakness. Similar benefits are seen with correction of metabolic acidosis, generally with sodium bicarbonate, which also helps to improve hyperkalemia. This electrolyte disorder is very prevalent in advanced CKD and may be difficult do manage. Drugs that increase potassium levels may need to be discontinued, optimizing diuretic dosage, and use of cation exchange resins are frequently necessary. When prognosis is poor or the last days are approaching, a symptom management alone to provide comfort may be a choice. Medications that do not meet this goal should be stopped (statins, aspirin, vitamin supplements and others) and medications to treat symptoms should be started as discussed in the next section. Ideally, an expert in palliative care should be involved20-35.
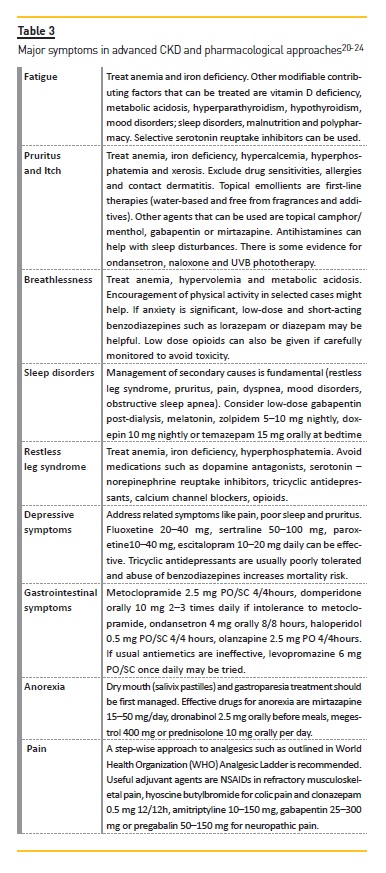
Active symptom management
Advanced CKD is associated with a multiplicity of symptoms which frequently occur in complex clusters, being difficult to treat in isolation22. Most are underrecognized, partly because many of them are from comorbid conditions and partly because there is a tendency to focus on biochemical markers and kidney management instead of patient complaints22. Most frequent symptoms are fatigue, pruritus and itch, breathlessness/shortness of breath, sleep disorders, restless leg syndrome, depressive symptoms, gastrointestinal symptoms, anorexia and pain. Non-pharmacological approaches should be considered first. Some examples that can improve overall symptoms are cognitive and behavioral therapy, relaxation therapy, aerobic and resistance exercises, sleep hygiene and avoidance of stimulants. Table 3 provides examples of a pharmacological approach to these symptoms.
Geriatric syndromes may be associated with earlier initiation of dialysis therapy (symptoms attributed to uremia) with loss of functional autonomy and increased mortality. The most frequent geriatric syndromes in ESRD are frailty, cognitive impairment, depression, falls and dependency in transfer25. Early identification and optimization of clinical condition is important before a final decision is made about whether or not to start dialysis25.
Regular symptom assessment using validated tools helps to redirect the treatment. There are eight validated tools to assess global symptom burden in CKD patients, with standouts the Edmonton Symptom Assessment System-Revised: Renal, the Palliative Care Outcome Scale- Renal and the Dialysis Symptom Index20. A meaningful improvement after active symptom treatment has been defined as a 30% improvement in scores22.
KDIGO recommends a stepwise approach for symptom management: nonpharmacologic interventions as a first line strategy and then further progress to pharmacologic treatments. Exercise and cognitive behavioral therapy had good results in improvement of sleep disorders, restless leg syndrome, fatigue, pain and depression22. Low-dose gabapentin and antidepressantsmay additionally improve these symptoms22.
Given the interrelated nature of symptom, an overall symptom approach is like to improve quality of live even if each symptom has not completely resolved. For example, a reduction in pain may improve sleep, humor and ability to cope with disease22. Consideration should be given to low-dose therapies that may be effective across several symptoms20.
Shared-Decision Making Process
Following the palliative care principles, KDIGO defines the shared-decision-making process as a process of communication by which physicians and patients agree on a specific course of action based on a common understanding of the patients treatment goals, taking into account the benefits and harms of treatment options and the likelihood of achieving the outcomes that are most important to individual patients. Because patients health status preferences and treatment options may change over time, shared decision making requires a flexible approach of reevaluation and redirection to ensure that the goals of care and treatment plans remain aligned with patients values and preferences20.
Schell et al.25 has proposed a guide for nephrologists regarding communication and decision making in advanced kidney disease named SPIRES (setup, perceptions and perspectives, invitation, recommendation, empathize, summarize and strategize).
The setup process (preparing for the conversation) is one of the most important stages. It begins with a review of patient´s record for pertinent clinical and laboratory information, followed by the use of prognostic tools to identify patients who may benefit from conservative management.
Prognostic scores can improve accuracy of the prognostic estimates, facilitate informed consent and support the discussion of supportive care goals26,27.
In a small study, patients considering dialysis initiation were willing to trade survival time gained in exchange for greater independence and less time in the health care system27. Current prognostic tools are not sensitive or specific enough to tell how long the patient will live but are informative at identifying high-risk patients8,21. The Renal Physician Association guidelines suggests that patients with 2 or more of the following factors are at high-risk for adverse outcomes with dialysis: age ≥ 75 years, a high Charlson Comorbidity index (≥ 8), poor functional status or disability (e.g. Karnofsky Performance status < 40) and severe malnutrition (serum albumin < 2.5 g/dL).
In recent years, several tools to predict short-term mortality on dialysis and need of kidney supportive care have been developed8,28-32 and are given in Table 4.
Potential new predictors for incorporation in prognostic scores such as potassium, phosphorus, blood pressure, interdialytic weigh gain, SF-36 physical and mental component score and Karnofsky Index are being explored because of their dynamics in the terminal months8.
Physicians clinical experience and judgment may also contribute to identification of high-risk patients.
The use of the Surprise Question: Would I be surprised if this patient died in the next year? is a validated and easy-to use screening tool. A recent survey of 300 patients with CKD stage 4 to 5 not on dialysis reveled that those who had no as an answer died more than 5 times comparatively to those who answer was yes33.
In a study with 150 hemodialysis patients, the answer no was associated with a 3.5 times higher mortality than the yes group34.
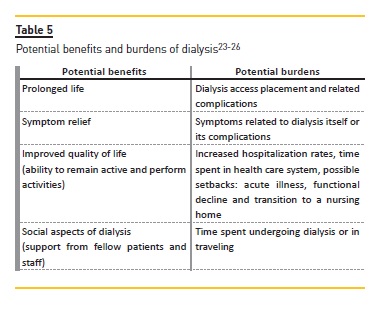
An understanding of burdens and benefits of dialysis, considering not just if the patient will survive on dialysis but how the patient will cope with undergoing treatments is also important (Table 5)25. After preparing relevant prognostic information, seeking other physicians opinions about significant illnesses (heart failure or cancer) can strengthen the information about prognosis and should be encouraged because it helps the nephrologist to understand how likely dialysis is to affect the patient´s overall course25. Table 6 summarizes the major principles of the SPIRES approach25.
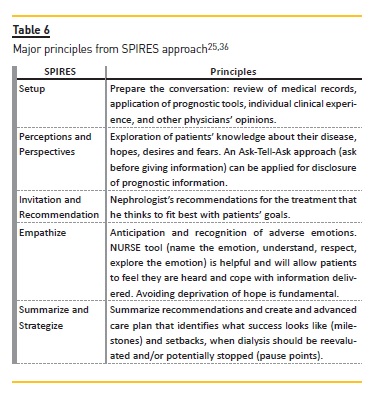
Early recognition and timely implementation of a management strategy are the mainstreams of advanced care planning (ACP). A time-limited trial of dialysis can be applied if there is doubt regarding how the patient may do on dialysis25. If there is a positive change in quality of life and health status, it is advantageous to continue with dialysis. However, if is not, treatment suspension must be considered.
Periodically and scheduled reevaluations are important to reassess if patients goals are being obtained.
Illness trajectories are different from patient to patient. Recently, Xie et al.37 identified 3 phenotypically distinct functional trajectories in 26,246 patients with CKD stage 4 in a five-year follow-up study: consistent slow decline (trajectory 1), consistent fast decline (trajectory 2) and early nondecline and late fast decline (trajectory 3).
Compared with those with consistent slow decline, patients with consistent fast decline had similar risk for kidney outcomes but significantly increased risk of death. Those with early nondecline and late fast decline were at higher risk for death than for kidney disease outcomes.
This highlights the necessity for discussion of possible scenarios in which patients would want to withhold or withdraw dialysis, which are fundamental to avoid potentially unwanted suffering and overuse of limited health care resources20. The illness trajectory of declining patients includes sentinel events like acute illness or loss of functional independence that signals the need to reassess care goals25,35. These pause points are also opportunities to address burdens that may benefit from specialized services, such as palliative care for ongoing symptom management25.
Holistic Approach
ACP should be developed using an ethical and holistic approach considering all aspects of patients life (family, social, cultural and spiritual dimensions) besides medical condition. This implies strict collaboration between nephrologists, palliative care physicians, nutritionists, nurses, and other professionals like social workers, psychologists or priests.
KDIGO recommends that a multidisciplinary team should deliver comprehensive conservative care and the composition of a multiprofessional team should include:1 nephrologist/nurse/psychosocial worker/counselor or psychologist/dietician/allied health professionals/chaplain;2 family doctors/community staff/health-care volunteers, and3 integration and/or liaison with specialist supportive care, according to country and region20. Additional efforts are needed to construct these teams and to implement adequate care20,25,35-39.
EVIDENCE REGARDING SURVIVAL IN CONSERVATIVE CARE
Evidence is limited and the range of alternative terms used prevented systematic studies. Also, practically there are no randomized, controlled trials (ethically and practically very difficult) and the remaining studies differ regarding age profile, comorbidity burden, how conservative/dialysis decisions were made, time from which survival is measure, patterns of referral and dialysis management21.
The key evidence can be distilled from a group of studies summarizes in a systematic review by OConnor and Kumar38. The median survival of those managed conservatively varied from 6 to 23 months. Survival was 65% at 1 year (median 1.95 years) for 75 patients managed conservatively (mainly because of lack of expected survival benefit). After 2 years, 60% had been managed without hospitalizations and the majority (71%) of deaths occurred at home.
A recent five-year prospective observational study compared 122 patients on conservative treatment with 273 patients who attended predialysis clinic (92 of whom underwent dialysis after an average of 9 months).
Patients on conservative management were significant older, with higher prevalence of dementia, peripheral vascular disease and two or more comorbidities (57% versus 40%, p<0.05) and malnourishment (66% versus 37%, p<0.05). Initial eGRF was similar between groups (MDRD formula). Mean adjusted patient survival was 20 months versus 33 months in the predialysis group (p<0.01). Median survival was 16 months for those managed conservatively and 32% survived more than 12 months after eGRF fell under 10 ml/min per 1.73m2. The major cause of death was the renal disease (62%), followed by cardiac disease (13%) and malignancies (12%). There was no significant difference between all dialysis patients and the group managed conservatively when analysis was restricted to patients aged > 75 years who had two or more comorbidities, at least one of which was congestive heart failure or ischemic heart disease39.
Similar findings were found in a large observational study, with no survival benefit for patients > 80 years and for those with lower performance scores16.
Chandna et al40 again reported a survival advantage to dialysis (mean survival of 21.2 versus 67.1 months, p<0.05), with patients managed conservatively being older and sicker. For patients > 75 years, the survival advantage of dialysis reduced to nonsignificant 4 months when corrected for age, high comorbidity and diabetes.
In a group of patients treated with PD, similar findings were also reported. The survival benefit of PD was not observed in those with high burden of comorbidities and functional impairment41.
EVIDENCE ON SYMPTOMS AND QUALITY OF LIFE IN CONSERVATIVE CARE
There is also limited evidence. Systematic review by OConnor and Kumar38 again provides good evidence about symptom burden and quality of life. All studies included reported significant symptom burden in those managed conservatively, especially in the last month.
Da Silva-Gane et al.42 evaluated quality of life every 3 months for up to 3 years in patients with CKD late stage 4 or early stage 5 managed conservatively or by dialysis. Patients on conservative care maintained quality of life and those who started dialysis, life satisfaction decreased significantly. Only 39% of 3702 nursing home ESRD patients with functional impairment maintained baseline function at 3 months after dialysis initiation, decreasing to 13% at one year43.
Brown et al.39 suggests that renal supportive care can be effective in ameliorating symptoms and maintaining quality of life. Patients treated with dialysis spend more time in hospital and that could have a negative impact on quality of life.
Carson et al45 showed that every day of additional survival was almost at the expense of a day spent in health care institutions (hospitalization:0.069 versus 0.043 hospital days per patient days survived).
Smith et al.14 revealed that 65% of deaths among those on dialysis occurred in the hospital compared with 27% of those managed conservatively.
POLICIES TO PROMOTE CONSERVATIVE AND PALLIATIVE CARE IN ESRD
In Europe, including Portugal, there is an urgent need to strength and develop policies and health care reforms to promote comprehensive conservative and palliative care in ESRD. As an example, a recent survey regarding nephrologists perceptions about dialysis withdrawal and palliative care in Europe45 reported that 74% had no specific training in these matters. Tamura et al.46 identified three major barriers to implementation of a specialized program: access to specific care, capacity to provide it and financial support. The author also suggested five general policies to address these barriers. Universal screening for comprehensive conservative and palliative care needs by nephrologists or other health care providers, with use of validated prognostic and symptom assessment tools previously discussed and documentation of the advanced care plan or surrogate decision maker in the medical record can overcome major access barriers. Training a nephrology workforce to deliver these types of care, along with nurses and other health care providers could be possible through fellowship programs, accreditation organizations and professional societies.
Enhancement of conservative and palliative care content and assessment of competencies in nephrology fellowship curriculum would also be important. Monetary funds are fundamental to support the creation of a structured and specialized program with a multidisciplinary team. Implementation of sharedsavings model, reimbursement for time-intensive services such as advanced care planning or homebased visits and research collaborations with ESRD multi-institutions could be valuable options46.
CONCLUSIONS
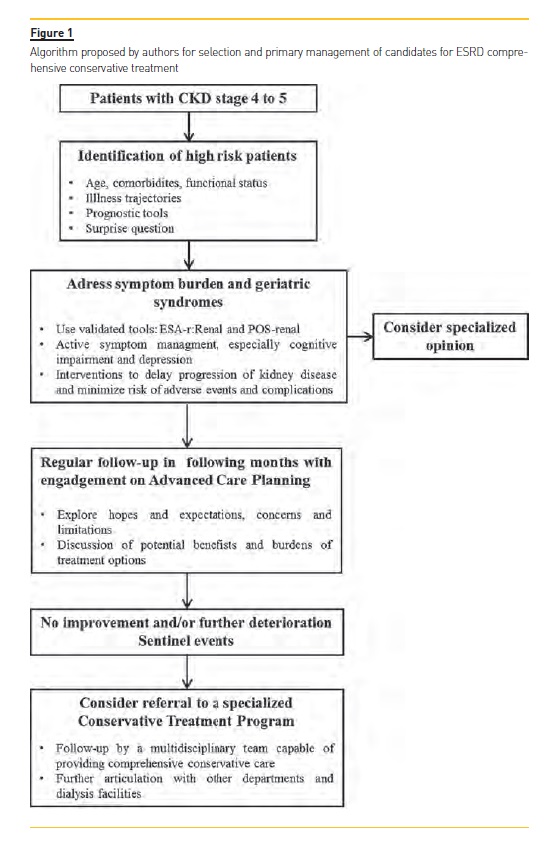
ESRD is becoming an increasingly geriatric condition and the option for a nondialytic management is increasingly recognized and delivered. Comprehensive conservative care has only been recently defined, allowing a holistic, standardized and stepwise approach for patients who will not do well on dialysis. Evidence suggests that, despite a potentially lower survival, benefits in quality of life and preservation of function outweighs those of dialysis and skills of palliative medicine helps to provide a reasonable symptom control. Patients managed conservatively also consume less health resources which could bring a positive effect in the countrys economy. Comprehensive conservative care should be recognized as a core competency and nephrology community should actively support and participate in research to address knowledge gaps and advocate policy changes. The creation of a multidisciplinary team and a specialized medical orientation are major present and future issues in order to optimize outcomes and meet patients goals. Considering all that was discussed in this article, the authors propose an algorithm (figure 1) that could be applied for selection and primary management of candidates for ESRD comprehensive conservative treatment.
References
1. Hazzan AD, Halimski C, Agoritsas S, Fishbane S, DeVita MV. Epidemiology and challenges to the management of advanced CKD. Adv Chronic Kidney Dis 2016; 23(4): 217-21. [ Links ]
2. Hill NH, Fatoba ST, Oke JL, Hirst JA, O´Callaghan CA, Lasserson DS, et al. Global prevalence of Chronic Kidney Disease – a Systematic Review and Meta-Analysis. PLoS One. 2016 Jul 6;11(7):e0158765. [ Links ]
3. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385: 117-71. [ Links ]
4. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743-800. [ Links ]
5. Murray CJL, Barber RM, Foreman KJ, et al. Global, regional, and national disabilityadjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet 2015; 386: 2145-91. [ Links ]
6. http://global-disease-burden.healthgrove.com/l/67121/Chronic-Kidney-Disease-in-Portugal. [Accessed 19 April, 2017] [ Links ]
7. Morton RL, Tamura MK, Coast J, Davison SN. Supportive care: economic considerationsin advanced kidney disease. Clin J Am Soc Nephrol 2016; 11(10): 1915-20. [ Links ]
8. Couchoud C, Hemmelgarn B, Kotanko P, Germain MJ, Moranne O, Davidson S. Supportive care: time to change our prognostic tools and their use in CKD. Clin J Soc Nephrol 2016; 11(10):1892-1901; [ Links ]
9. Cohen LM, Ruthazer R, Moss AH, Germain MJ. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol 2010; 5(1): 72-9. [ Links ]
10. Berger JR, Hedayati SS. When a conservative approach to advanced chronic kidney disease is preferable to renal replacement therapy? Seminars in Dialysis 2015; 27(3):253-6. [ Links ]
11. Noordzij M, Jager K. Increased mortality early after dialysis initiation: a universal phenomenon. Kidney Int 2014; 85(1): 12-4. [ Links ]
12. Bradbury BD, Fissel RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2007; 2: 89-99. [ Links ]
13. Murtagh FEM, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transpant 2007; 22: 1955-62. [ Links ]
14. Smith C, Da Silva Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyze: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract 2003; 95: c40-6. [ Links ]
15. Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4: 1611-9. [ Links ]
16. Hussain JA, Mooney A, Russon L. Comparison of survival analysis and palliative care involvement in patients over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med 2013; 27(9): 829-39. [ Links ]
17. Tamura MK, Covinsky KE, Chertow GM, Yaffe K, Landefeld S, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361(16): 1539-47. [ Links ]
18. Germain MJ, Davison SN, Moss AH. When enough is enough: the nephrologist´s responsibility in ordering dialysis treatments. Am J Kindey Dis 2011; 58(1): 135. [ Links ]
19. Coelho A, Diniz A, Hartz Z, Dussaultd G. Gestão integrada da doença renal crónica: análise de uma política inovadora em Portugal. Ver Port Saúde Pública 2014; 32(1): 69-79. [ Links ]
20. Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brenann F, et al. Executive Summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int 2015; 88(3): 447-59. [ Links ]
21. Murtagh FEM, Burns A, Moranne O, Morton RL, Naicker S. Supportive care: comprehensive conservative care in end-stage kidney disease. Clin J Am Soc Nephrol 2016;11(10): 1909-14. [ Links ]
22. Davison SN, Jassal SV. Supportive care: integration of patient-centered kidney care to manage symptoms and geriatric syndromes. Clin J Am Soc Nephrol 2016; 11(10): 1882-91. [ Links ]
23. Vandecasteele SJ, Tamura MK. A patient-centered vision of care for ESRD: dialysis as a bridging treatment or as a final destination? J Am Soc Nephrol 2014; 25: 1647-51. [ Links ]
24. Grubbs V, Moss AH, Cohen LM, Fischer MJ, Germain MJ, Jassal V, et al. A palliative approach to dialysis care: a patient centered transition to the end of life. Clin J Am Soc Nephrol 2014; 9(12): 2203-9.
25. Schell JO, Cohen RA. A communication framework for dialysis decision-making for frail elderly patients. Clin J Am Soc Nephrol 2014; 9: 2014-21. [ Links ]
26. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed. Rockville, MD: Renal Physicians Association; 2010. [ Links ]
27. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 195-204. [ Links ]
28. Thamer M, Kaufman JS, Zhang Y, Zhang Q, Cotter DJ, Bang H, et al. Predicting early death among elderly dialysis patients: Development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis 2015; 66: 1024-32. [ Links ]
29. Couchoud CG, Beuscart JB, Aldigier JC, Brunet PJ, Moranne OP. REIN Registry: Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly with end-stage renal disease. Kidney Int 2015; 88: 1178-86. [ Links ]
30. Couchoud C, Labeeuw M, Moranne O, Allo V, Esnault V, Frimat L, et al. French Renal Epidemiology and Information Network (REIN) registry: A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant 2009; 24: 1553-61. [ Links ]
31. Mauri JM, Vela E, Clèries M. Development of a predictive model for early death in diabetic patients entering hemodialysis: A population-based study. Acta Diabetol 2008; 45: 203-9. [ Links ]
32. Cohen LM, Ruthazer R, Moss AH, Germain MJ. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol 2010; 5: 72-9. [ Links ]
33. Javier AD, Figueroa R, Siew ED, Salat H, Morse J, Stewart TG, et al. Reliability and utility of the surprise question in CKD stages 4 to 5. Am J Kidney Dis 2017; pii: S0272-6386(17)30006-9 [ Links ]
34. Moss AH, Ganjoo J, Sharma S, Gansor J, Senft S, Weaner B, et al. Utility of the surprise question to identify patients with high mortality. Clin J Am Soc Nephrol 2008; 3: 1379-84. [ Links ]
35. Koncicki HM, Schell JO. Communication skills and decision making for elderly patients with advanced kidney disease: a guide for nephrologists. Am J Kidney Dis 2016; 67(4):688-95. [ Links ]
36. Davison SN, Torgunrud C. The creation of an advanced care planning process for patients with ESRD. Am J Kidney Dis 2007; 49(1): 27-36. [ Links ]
37. Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z. Estimated GFR trajectories of people entering CKD stage 4 and subsequent kidney disease outcomes and mortality. Am J Kidney Dis 2016; 68(2): 219-28. [ Links ]
38. O´Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med 2012; 15: 228-35. [ Links ]
39. Brown MA, Collet GK, Josland EA, Foote C, Li Q, Brennan FP. CKD in elderly patients managed without dialysis: survival, symptoms and quality of life. Clin J Am Soc Nephrol 2015; 10: 260-8. [ Links ]
40. Chandna SM, Da Silva-Gane M, Marshalll C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 2011; 26: 1608-14. [ Links ]
41. Shum CK, Tam KF, Chak WL, Chan TC, Mak YF, Chau KF. Outcomes in older adults with stage 5 chronic kidney disease: comparison of peritoneal dialysis and conservative management. J Gerontol A Biol Sci Med Sci 2014; 69: 308-14. [ Links ]
42. Da Silva-Gane M, Wellsted D, Greenshields H, Norton S, Chandna SM, Farrington K. Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol 2012; 7: 2002-9. [ Links ]
43. Kurella TM, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539-47. [ Links ]
44. Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4: 1611-19. [ Links ]
45. Biesen WV, Lujtgaarden WM, Brown EA, Michel JP, Munster BC, Jager KJ, et al. Nephrologists perceptions regarding dialysis withdrawal and palliative care in Europe: lessons from a European Renal Best Practice survey. Nephrol Dial Transplant 2015; 30(12): 1951-58. [ Links ]
46. Meier DE, Tamura MK. Five policies to promote palliative care for patients with ESRD. Clin J Am Soc Nephrol 2013; 8: 1783-90. [ Links ]
Carolina Lã Belino, MD
Department of Nephrology, CHVNG/E
Rua Conceiçã o Fernandes 4434-502,Vila Nova de Gaia, Portugal
E-mail: carolinabelino@hotmail.com
Support: No financial or non-financial support of any kind was provided.
Disclosure of potential conflicts of interest: none declared.
Received for publication: Nov 11, 2017
Accepted in revised form: Mar 12, 2018














