Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.2 Lisboa jun. 2018
CASE REPORT
Pediatric pulmonary embolism and nephrotic syndrome
Raquel Costa1, Susana Gomes1, Rosário Stone2, Rosário Ferreira3
1 Pediatrics Department. Hospital Espírito Santo de Évora
2 Nephrology and kidney transplantation Unit. Pediatrics Department. Hospital de Santa Maria. Centro Académico de Medicina de Lisboa
3 Pneumology Unit. Pediatrics Department. Hospital de Santa Maria. Centro Académico de Medicina de Lisboa
ABSTRACT
Thromboembolic complications, such as pulmonary embolism, are well-documented complications in nephrotic syndrome. The authors report a case of a 10-year-old boy with nephrotic syndrome and history of thrombotic complications, who experienced a massive pulmonary embolism during nephrotic syndrome relapse. Clinical signs and symptoms were minimal; D-dimers were an important clue and computed tomography pulmonary angiography established the definitive diagnosis. Treatment with low weight heparin was effective. Screening for hereditary thrombophilia identified predisposition towards thrombosis. Although these events seem rare in children, the literature suggests that they are actually underdiagnosed. Clinicians should be aware of this clinical condition, in order to avoid delay or misdiagnosis and prevent a poor or fatal outcome.
Keywords: hypocoagulation, nephrotic syndrome, pediatrics, pulmonary embolism.
INTRODUCTION
Nephrotic syndrome (NS) is associated with an increased risk of thromboembolic complications secondary to a multifactorial hypercoagulable state that involves abnormalities in coagulation factors, anticoagulant proteins, fibrin formation and platelet aggregation. Other factors such as the use of diuretics, steroids therapy, past history of embolic event and documented inherited thrombophilia can contribute to increasing the current thromboembolic risk1-8. Thromboembolic events can occur on different sites, in both venous and arterial territories. The territories more frequently involved in vascular thrombosis are the cerebral venous sinuses and middle cerebral arteries, deep veins of the lower limbs, vena cava, renal and hepatic veins and mesenteric artery1-8.
In childhood these events can be silent, leading to a delay or misdiagnosis, which may result in a catastrophic outcome1,2.
This case report describes the subtle presentation of a massive pulmonary embolism (PE) in a boy with steroid-dependent NS.
CASE REPORT
The authors report the case of a 10-year-old boy with a 7-year history of steroid-dependent NS with multiple relapses, treated with low-dose prednisolone Kidney biopsy showed diffuse membranoproliferative glomerulonephritis. Patient history was significant for cerebral venous sinus thrombosis at the age of four, presented as severe headache, during a nephrotic syndrome relapse (protein/creatinine ratio 13.1 mg/mg; albumin 0.7 g/dL). This thrombotic event had a favorable evolution, without sequelae. Investigation after the episode did not include screening for additional risk factors, such as screening for inherited thrombophilia.
At the age of 10, the patient was admitted for weight gain of 2 kg and edema of the extremities associated with intermittent episodes of chest pain and shortness of breath that resolved spontaneously.
On physical examination, the patient had Cushingoid appearance and presented with minor edema at the extremities, tachypnea (respiratory rate 36/min). Pulmonary auscultation showed reduced breath sounds in both pulmonary bases. Temperature was 36.6oC, blood pressure 105/70 mmHg, heart rate 80–90 bpm and oxygen saturation 97% (at room air).
Urinalysis showed 4+ of protein on dipstick and urinary protein/creatinine ratio 26.7 mg/mg. The blood work revealed estimated filtration rate 110 mL/min/1.73m2, albumin 1,6 g/dL, hemoglobin 15.8 g/dL, hematocrit 45%, platelet count 564000/μL, total cholesterol 444 mg/dL and elevated D-dimer test 9.98 μg/mL [< 0.5 μg/mL]. Arterial blood gas on room air presented acute uncompensated respiratory alkalosis (pH 7.47, pCO2 34 mmHg, pO2 78 mmHg, HCO3- 26.1mmol/L).
Chest X-ray showed bilateral small pleural effusion. Electrocardiogram and echocardiogram showed noabnormal findings.
Computed tomography pulmonary angiography (CTPA) documented thromboembolism in both right and left branches of the pulmonary artery, with complete occlusion on the left side, associated with an area of pulmonary infarction (Fig. 1 and 2). Ventilation/perfusion scan revealed major impairment of ventilation/perfusion in the left lung, with global pulmonary function sustained at 94% in the right lung.
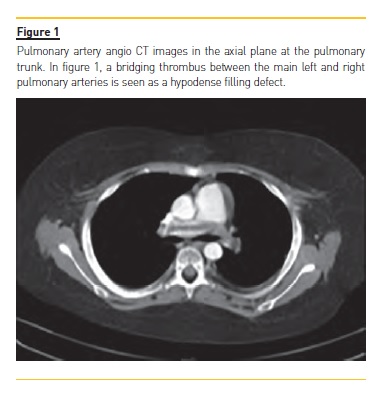
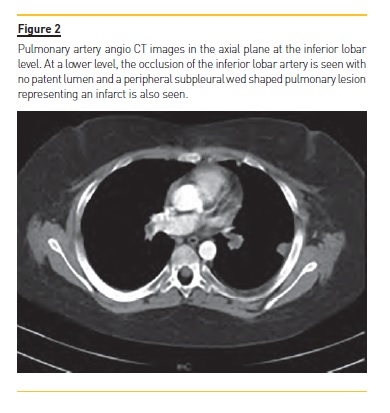
To treat the NS relapse, the patient incremented his prednisolone dose to 60 mg/m2/day. He received 1 administration of 20% human albumin solution infusion.
Anticoagulation with low weight heparin (1 mg/kg/dose BID) was started. The NS remitted after 20 days of treatment. Pulmonary embolism showed a favorable evolution, with complete resolution documented by computed tomography 4 weeks after the event. Two doses of Rituximab were administered on the 40th and 45th day after the thrombotic episode, along with MMF (1200 mg/m2/day). Prednisolone dose was progressively reduced (minimum dose of 10 mg/day). Hypercholesterolemia due to nephrotic syndrome persisted after remission, so simvastatin was maintained. Anticoagulation with low weight heparin was maintained for 6 months (1 mg/kg/dose BID).
Screening for hereditary thrombophilia showed heterozygosity for the thermolabile variant C677T of the methylenetetrahydrofolate reductase and heterozygosity for the plasminogen activator inhibitor gene.
The patient remained relapse-free for 12 months after the episode. During the subsequent relapse, he received prednisolone (60 mg/m2/day) and daily prophylactic dose of enoxaparin. Remission occurred within 7 days of treatment.
During the 4-year follow-up there was no record of other thrombotic events. His pulmonary function is normal.
DISCUSSION
NS is associated with an increased risk of thromboembolic complications3-8. The incidence of thrombotic events is lower in children (3%) than in adults (25%)4.
However, Hoyer et al concluded that these complications in childhood are actually underdiagnosed9,10 and others have suggested that the chance of such complications is higher if multiple risk factors are present3-8.
Although pulmonary embolism is rare in children, it can be fatal. Therefore, the authors highlight the importance of recognizing its clinical features, such as minor respiratory symptoms, and the high level of suspicion needed to establish an early diagnosis and provide an adequate treatment, in order to allow a favorable outcome.
The patient described herein had a pulmonary embolism during a NS relapse; he presented with high urinary protein/creatinine ratio and low plasma albumin, in accordance with what is described as most likely in literature3,4. The use of plasma D-dimers have little evidence in children, but, since the high levels of D-dimers are markers for thrombosis, in this case provide an important clue to the diagnosis. CTPA established a definitive and reliable diagnosis of pulmonary embolism5.
Furthermore, the patient history was positive for sinus thrombosis, which increases the chance of additional events3. For this reason, screening for inherited thrombophilia should be requested after the first thrombotic event11. In this case report, mutations that reveal a predisposition towards thrombosis were identified.
In patients with a recurrent but reversible risk factor for thrombotic events, the literature suggests the use of prophylactic anticoagulation at times of risk factor recurrence and until its resolution, such as in cases of NS relapse13. Accordingly, taking patient history into consideration, prophylactic hypocoagulation therapy was started based on individual risk-benefit during relapses3,5-7,12-14.
Chronic steroid therapy in patients with steroid dependent or frequently relapsing NS is associated with numerous side effects. As such, steroid-sparing therapies such as alkylating agents, calcineurin inhibitors, levamisole, mycophenolate mofetil and rituximab are used as an alternative treatment course to reduce the risk of relapse. There is no preferred method of treatment supported by the literature, and the choice is based on clinical experience and potential side effects. In this patient, the steroidsparing medication used was MMF in addition with Rituximab, that has been shown to be effective in sustaining remission.15-17
CONCLUSION
PE is a known but rare complication of NS in children.
These events can be silent, leading to a delay or misdiagnosis.
As such, clinicians should saying of what to start adequate treatment and prevent a poor or fatal outcome. Screening for inherited thrombophilia is required after the first event. Anticoagulation in the pediatric population can be controversial, but it should be started during relapse in these patients.
References
1. Jones CL, Hebert D (1991) Pulmonary thrombo-embolism in the nephrotic syndrome. PediatrNephrol5:56-58. [ Links ]
2. McPheeters A, Thomas P, Snyder D, Peterson R (2003) Fatal pulmonary embolism in a 10-year old with nephrotic syndrome. Cal J Emerg Med 4(2):36-38. [ Links ]
3. Kerlin BA, Blatt NB, Fuh B, Zhao S, Lehman A, Blanchong C, et al. (2009) Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: a Midwest Pediatric Nephrology Consortium (MWPNC) study. J Pediatr 155(1):105-110. [ Links ]
4. Kerlin BA, Ayoob R, Smoyer WE (2012) Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 7(3):513-520. [ Links ] [ Links ]
6. Mahmoodi B, Kate M, Waanders F, Veeger N, Brouwer J, Vogt L, et al. (2008) High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: Results from a large retrospective cohort study. Circulation 117(2):224-230. [ Links ]
7. Biss TT, Brandão LR, Kahr WH, Chan AK, Williams S (2008) Clinical features and outcome of pulmonary embolism in children. Br J Haematol 142(5):808-818. [ Links ]
8. Bülyükçelik M, Karakök M, Başpinar O, Balat A (2008) Arterial thrombosis associated with factor V Leiden and methylenetetrahydrofolate reductase C677T mutation in childhood membranous glomerulonephritis. Pediatr Nephrol 23(3):491-494. [ Links ]
9. vanOmmen CH, Heijboer H, Groothoff JW, Teeuw R, Aronson DC, Peters M (1998) Persistent tachypnea in children: keep pulmonary embolism in mind. J Pediatr Hematol Oncol 20(6):570-573. [ Links ]
10. Hoyer PF, Gonda S, Barthels M, Krohn H, Brodehl J (1986) Thromboembolic complications in children with nephrotic syndrome. Acta Paediatr Scand 75:804-810. [ Links ]
11. Pereira JR, Leite F, Pio D, Morais L, Costa T, Faria MS (2011) Tromboembolismo pulmonar no síndrome nefrótico associado a trombofilia. Nascer e Crescer 20(4):262-265. [ Links ]
12. Deshpande P, Griffiths M (2005) Pulmonary thrombosis in steroid-sensitive nephrotic syndrome. PediatrNephrol 20(5):665-669. [ Links ]
13. Monagle P, Chan AKC, Goldenberg NA, et al. (2012) Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 141(2) Suppl:e737S-e801S. [ Links ]
14. Lilova M, Velkovski I, Topalov I (2000) Thromboembolic complications in children with nephrotic syndrome in Bulgaria (1974–1996). Pediatr Nephrol 15:74-78. [ Links ]
15. Benz MR, Toenshoff B, Weber LT (2014) Treatment of children with frequently relapsing steroid-sensitive nephrotic syndrome: recent trial results. Clin Investig (Lond) 4(11):1043-1054. [ Links ]
16. Hogg RJ, Fitzgibbons L, Bruick J, et al. (2006) Mycophenolate mofetil in children with frequently relapsing nephrotic syndrome: a report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol 1(6):1173-1178. [ Links ] [ Links ]
Raquel Costa, MD
Hospital Espírito Santo de Évora, Largo Senhor da Pobreza
7000-811, Évora, Portugal
E-mail: raquelfreitascost@gmail.com
ACKNOWLEDGEMENTS
The authors would like to acknowledge Vasco Heredia for commenting on medical imaging studies and for the valuable suggestions on this manuscript.
Disclosure of potential conflicts of interest: there are no known conflicts of interest associated with this publication and there has been no financial support for this work that could have influenced its outcome.
Received for publication: Dec 10, 2017
Accepted in revised form: Mar 10, 2018














