Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.4 Lisboa dez. 2018
ORIGINAL ARTICLE
Are peritoneal protein losses related to peritonitis risk in patients on peritoneal dialysis?
Sara Daniela Rodrigues, Maria Clara Santos, Ana Marta Gomes, João Carlos Fernandes
Serviço de Nefrologia, Centro Hospitalar Vila Nova de Gaia-Espinho, Vila Nova de Gaia, Portugal
ABSTRACT
Background: Peritoneal protein losses (PPL) are an inevitable process in peritoneal dialysis (PD). Few studies have supported a positive correlation between PPL and infections or general morbidity and mortality. The aim of this study was to investigate whether PPL was a risk factor of peritonitis.
Methods: We retrospectively studied all incident PD patients in a PD single unit during the previous 10 years. We recorded baseline PPL (bPPL) and relevant analytic and clinical data. Patients were distributed into one of 2 bPPL groups (group 1: ≤5.89g/day and group 2: >5.89g/day) to compare their peritonitis risk.
Results: 104 Patients were included, with a median follow‑up of 29 months. Higher bPPL patients were more likely to have peritonitis (group 1: 43%, group 2: 72%, p<0.01). After adjustment for covariates, bPPL remained an independent predictor of peritonitis (p=0.01). The time to first peritonitis was shorter in the group with higher bPPL (p=0.01) although after adjustment for other covariates no significant associations were found. In the Poisson regression, more elevated bPPL were associated with higher peritonitis rates after adjustment for other independent variables (p=0.02).
Conclusion: In this retrospective study, higher bPPL were able to independently predict risk of peritonitis, reflecting its impact on the morbidity of PD patients. Patients with higher bPPL levels presented shorter peritonitis‑free time to first peritonitis episode and higher probability of more peritonitis episodes over time.
Keywords: peritonitis, peritoneal dialysis, peritoneal protein losses
INTRODUCTION
Peritoneal protein losses (PPL) are an inevitable and permanent process in peritoneal dialysis (PD). In stable continuous ambulatory peritoneal dialysis patients, the amount of protein leakage has been described as around 5 g per 24 hours, 4 g of which is albumin1,2. Other proteins include transferrin, immunoglobulins, complement factors, β2‑microglobulin and β2‑macroglobulin.
However, there are marked inter‑and intra‑individual differences, depending on the number and diameter of their peritoneal pores2. Protein clearance is higher in the presence of peritonitis, probably due to an increase in peritoneal permeability and/or effective surface area3,4.
Patients with diabetes mellitus have also been reported as having increased peritoneal permeability and, subsequently, higher PPL, although there is uncertainty about the underlying mechanisms. Changes in basal membrane structure and thickness as well as arterial hyalinosis have been postulated5‑7.
Other associations with higher peritoneal protein clearance have been peripheral arterial disease and endothelial dysfunction in general8,9 and increased peritoneal membrane transport10.
However, only a few studies have supported a positive correlation between PPL and infections or general morbidity and mortality11‑16.
The aim of this study was to investigate whether baseline PPL (bPPL) was a risk factor of peritonitis in PD patients.
SUBJECT AND METHODS
We retrospectively studied all incident PD patients in our center (Centro Hospitalar Vila Nova de Gaia e Espinho, Portugal) from September 2007 to September 2017. Only patients with an initial peritoneal kinetics test performed within one month after the start of PD were included. We recorded relevant baseline clinical data, including age, gender, race, presence of diabetes mellitus, cerebrovascular disease, peripheral arterial disease, and analytic parameters, such as serum hemoglobin (Hb) and albumin and effluent characteristics, including protein losses (grams per 24 hours). Membrane transport status obtained in the first peritoneal equilibration test (PET) (dialysate‑to‑plasma creatinine [D/P Cr] ratio at 4 hours), was also recorded although, in relation to this particular test, data collected referred to 2 to 3 months after the onset of PD – time when this exam has routinely been done in our unit.
Throughout this period, biocompatible solutions were used, namely Physioneal®, from Baxter (Baxter Healthcare, Castlebar, Ireland), or Balance®, from Fresenius(Fresenius Medical Care, Bad Homburg, Germany), in a distribution of approximately 50% each.
In the Fresenius group, we have used Balance® bags, whose buffer is lactate. It was not possible to obtain specific information about the solutions used in each case during the follow‑up.
We know that dialysate containing glucose polymers (Icodextrin) were not exclusive to patients treated with the Baxter system; patients treated with the Fresenius system received it too when necessary through the use of an adapter.
PET was performed during a 4‑hour dwell with Physioneal® 3.86% glucose dialysate or with Balance® 4.25% glucose dialysate. First episode and total number of peritonitis were registered. Relapsing peritonitis were considered as single episodes. Other PD‑related infections and infection‑related catheter removal were reported. We also recorded non‑PD‑related infections requiring hospital admission, CV events and death, and other reasons to abandon the PD program.
Patients were distributed into 2 bPPL according to the bPPL median (group 1: ≤5.89g/day and group 2: >5.89g/day) to compare their peritonitis risk.
The results are presented as frequency (n) and/or percentage (%) for categorical variables, mean ± standard deviation (SD) for continuous normally distributed data, or median (interquartile range [25th to 75th percentile]) for non‑normally distributed continuous data. The characteristics of the 3 bPPL groups were compared using a chi‑square test, Mann‑Whitney and Wilcoxon tests, or ANOVA, depending on data type.
All variables with a statistical association with peritonitis of p<0.1 in the univariate analysis and those thought to be of clinical significance (age, sex, diabetes, peripheral arterial disease, membrane transport status, normalized protein nitrogen appearance [nPNA]) were used to create multivariate models in the logistic regression, to determine associations with bPPL. Time to first episode of peritonitis was estimated by univariate Kaplan‑Meier survival analysis (log‑rank test). Multivariate Cox proportional hazards models were built to analyze the predictive role of bPPL in the risk for first‑episode peritonitis, using the previously mentioned assumptions in the choice of covariates. Analyses were censored at death, transplantation, transfer to hemodialysis and loss of follow‑up.
Rates of peritonitis were compared between groups using univariate and multivariable Poisson regression. All data was analyzed using the SPSS Statistics 23 (IBM Corp, NY, USA). P values < 0.05 were considered statistically significant.
RESULTS
In total, 104 patients started PD in this period (Table I). The primary cause of chronic kidney disease (CKD) was chronic glomerulonephritis. Diabetes was present in less than a quarter of cases (Table I). The median follow‑up was 29(17‑47) months. 70 Patients left our PD program during the follow‑up, mainly for kidney transplantation (n=35) and secondarily to PD related infections (peritonitis n=12, peritoneal catheter exit‑site [ES] or tunnel infection n=3). Other causes were ultrafiltration failure and/or low clearance (n=5), surgical contra‑indication (n=5), loss of patients autonomy to perform the technique (n=4), presence of dialysate leak (n=3), patients choice for hemodialysis (n=2) and emigration (n=1). Finally, 7 patients died.
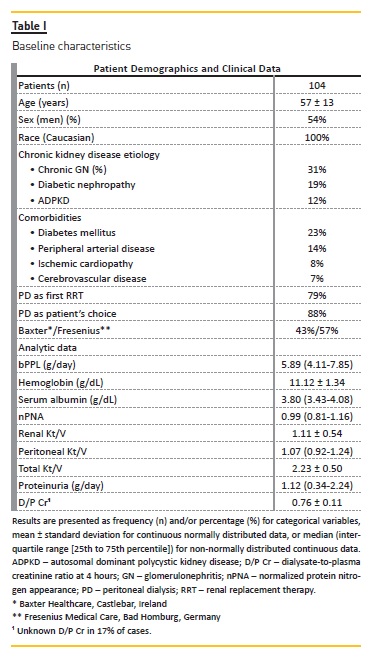
Baseline demographic and clinical characteristics were comparable between the 2 bPPL groups (Table II). Although in group 2 the median age was higher and with slightly greater male preponderance compared to group 1, these differences were not statistically significant.
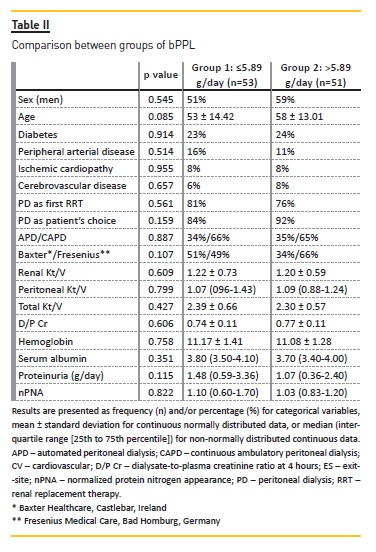
Over this time, 134 episodes of peritonitis were observed in 60 patients, with a maximum rate of 7 episodes/patient. Distribution of the number of peritonitis per group is described in Figure 1). We observed that more patients in group 1 presented no peritonitis than in group 2 (n=29 versus n=15), whereas more patients in group 2 had 1 (n=18), 2 (n=6) or 3 (n=6) peritonitis than in group 1 (n=11, n=4 and n=1, respectively).
Also, only patients in group 2 presented 7 episodes of peritonitis along the follow‑up (n = 2) (Figure 1, right side graphic). On the other hand, we observed that, in both groups, the same number of patients presented 4 (n=5) and 5 (n=1) peritonitis, noting that 5 of these cases were considered to be group 1 outliers (Figure 1, left side graphic). At the same time, we believe that the two patients in group 2 who presented 7 episodes of peritonitis might have also corresponded to outliers. Looking at these 7 patients with higher values of peritonitis, we observed that they were older (median age 60 years), with greater male preponderance (71%), lower median nPNA (0.83) and had slightly more heavy proteinuria (median 1.298g/day) at baseline. Considering these outliers as possible bias factors in our analysis, we excluded them whenever this allowed to obtain more robust results.
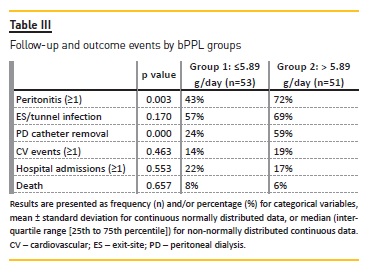
In univariate analysis, peritonitis and infection‑related catheter removal were more common in group 2 versus group 1 (Table III). ES and tunnel infections were also more common in the higher bPPL group, although without statistical significance. Besides bPPL, no other variables showed a statistically significant association (p<0.05) with peritonitis, or other PD‑related infections, in the univariate analysis. In the multivariable analysis, higher groups bPPL remained at higher risk to develop a first episode of peritonitis (odds ratio [OR] 4.02, p=0.01) (Table IV).
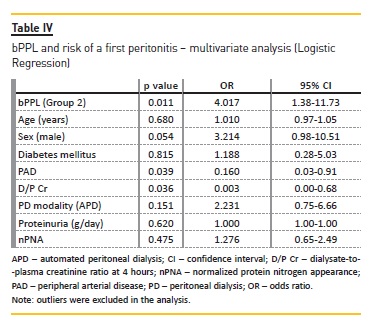
In the survival analysis, time to the first peritonitis was significantly shorter in group 2 (p=0.013) (Figure 2) and the overall time in PD in this group was inferior to that of the group 1 (p=0.01). This means that patients with higher bPPL had shorter peritonitis‑free time until the first peritonitis and that they remained for shorter periods of time on PD. However, after adjustment for covariates (Cox regression), no statistically significant associations were seen between peritonitis free‑time and bPPL.
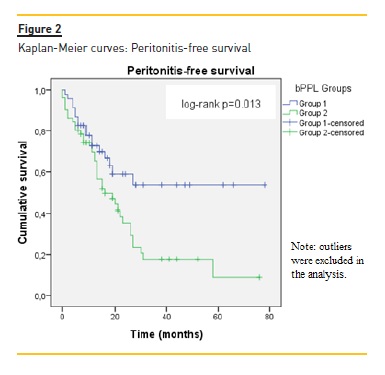
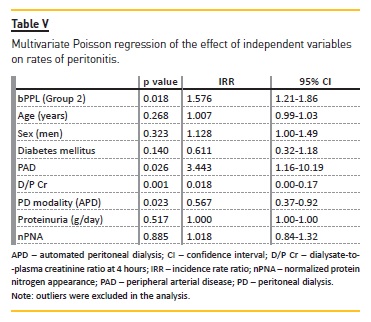
In the analysis of the effect of independent variables on rates of peritonitis (multivariate Poisson regression), bPPL was significantly associated with higher number of episodes during the follow‑up after adjustment for other relevant variables (p=0.02) (Table V). D/P Cr and peripheral arterial disease also related with higher peritonitis rates, but with less significant results.
No difference was observed in patient mortality or in the occurrence of severe non‑PD‑related infections between groups.
DISCUSSION
Nowadays, peritonitis rates are very high, approximately 1 episode in 30 patient‑months in several series16‑19, and it is extremely important that clinicians understand its risk factors.
We present here a retrospective study of the effect of baseline protein peritoneal excretion on the risk of peritonitis. Although PPL are a well‑known process in PD that have been described for over 30 years, the impact that greater protein losses can have on morbidity, including infections, on mortality or even on the preservation of the peritoneal membrane is not completely established as of yet. Likewise, we do not know if patients with higher losses should have a more differentiated follow‑up or even if something can be done to counteract this phenomenon.
We started by specifying that we would analyze PPL recorded at baseline, that is, at the beginning of PD, because that way we ensured that protein losses were altered as little as possible by transport changes induced by PD solutions, membrane deterioration over time or by peritonitis itself.
We found in our study several results supporting a positive association between peritoneal protein losses and peritonitis. Patients with higher bPPL presented higher risk of having peritonitis over time, shorter peritonitis‑free time and higher rates of peritonitis episodes.
We believe these results are valid, because, except for Cox regression, these effects maintained significant after adjustment for other pertinent variables and are in accordance with other previous reports4,11,12. It has been proposed that the predictive role of PPL in peritonitis is linked to systemic inflammation.
Nevertheless, the association between baseline peritoneal protein excretion and peritonitis has been shown to be independent of inflammatory makers4,12.
It has also been postulated that local inflammation in the peritoneal cavity, possibly related to vascular endothelial growth factor and leptin released by the local adipose tissue, leading to vasodilatation and intercellular fenestra, may contribute to the increased risk for peritonitis with high PPL, although this needs to be explored4,20,21. According to these hypotheses, PPL could simply represent a marker of local inflammation and a higher risk of peritonitis. On the other hand, it has been also hypothesized that the excreted proteins themselves can modulate the action of leukocytes and favor the onset of local infection, but there is very little research on this point22.
In addition, we observed a shorter time in PD in patients in higher bPPL groups. This could be explained by several reasons. First, and as expected, there was a greater need to remove the PD catheter owing to the higher rate of PD‑related infections in these patients and, therefore, an increased transfer to hemodialysis.
Second, these patients would have in theory a greater burden of morbidity11,12, and thus greater loss of autonomy and inability to perform the technique. Third, larger PPL would indicate greater endothelial dysfunction and would eventually be associated with faster membrane transport status, in turn associated with loss of efficient membrane transport over time and consequent need for technique transfer11,23. D/P Cr positive relationship with higher peritoneal protein clearance has been previously observed24,25. Here, however, we found no association between D/P Cr and bPPL. It should be noted that, in our sample, membrane transport status data did not have the same exact timing as bPPL and that 17% of patients had no available PET records. Together, this could have additionally compromised the statistical analysis concerning D/PCr. We found no association between higher PPL and an increased risk of CV events and mortality, as has been suggested previously9,14,15. Those associations have been attributed to higher morbidity and worse nutritional state, or even because depletion of specific proteins contribute to dyslipidemia, hormonal disturbances and a prothrombotic environment4,8. However, our sample was small and we cannot safely make conclusions about this point.
We recognize that there are limitations to our analysis. It is a retrospective study, with a limited statistical potency and there is an absence of data on inflammatory markers, the precise therapy applied during total follow‑up, or other potentially important clinical variables that could not be controlled. However, given the consistency of the main results, we believe that we can cautiously take some of them as valid.
In summary, our study shows a definite correlation between baseline PPL during PD and peritonitis. Quantification of baseline 24‑hour total dialysate protein content is a reproducible estimator of PPL and showed a significant association with the risk of peritonitis.
These data support that PPL should be monitored as a baseline marker of outcome in PD patients. In accordance with theoretical considerations, contemplating PPL either as a marker of vascular disease or as a pathogenic disorder are not mutually exclusive, and may help to explain the association between PPL and the outcome of PD patients.
References
1. Dulaney JT, Hatch FE Jr. Peritoneal dialysis and loss of proteins: a review. Kidney Int 1984; 26:253‑262.
2. Krediet RT, Zemel D, Imholz AL, Koomen GC, Struijk DG, Arisz L. Indices of peritoneal permeability and surface area. Perit Dial Int 1993; 13:S31‑S34. [ Links ]
3. Krediet RT, Zuyderhoudt FM, Boeschoten EW, Arisz L. Peritoneal permeability to proteins in diabetic and non‑diabetic continuous ambulatory peritoneal dialysis patients. Nephron 1986; 42(2):133‑140. [ Links ]
4. Dong J, Chen Y, Luo S, Xu R, Xu Y. Peritoneal protein leakage, systemic inflammation, and peritonitis risk in patients on peritoneal dialysis. Per Dial Int 2013; 33:273‑279. [ Links ]
5. Coronel F, Cigarrán S, Herrero JA, Delgado J, Ramos F, Gomis A. Peritoneal protein losses in diabetic patients starting peritoneal dialysis: is there a relationship with diabetic vascular lesions? Adv Perit Dial 2009; 25:115‑118. [ Links ]
6. Nakamoto H, Imai H, Kawanishi H, et al. Effect of diabetes on peritoneal function, assessed by personal dialysis capacity test in patients undergoing CAPD. Am J Kidney Dis 2002; 40:1045‑1054. [ Links ]
7. Parving H. Increased microvascular permeability to protein in short and long term juvenil diabetes. Diabetes 1976; 25(2):884‑889. [ Links ]
8. Sanchez‑Villanueva A, Bajo MA, Del PG, et al. Higher daily peritoneal protein clearance when initiating peritoneal dialysis is independently associated with peripheral arterial disease (PAD): A possible new marker of systemic endothelial dysfunction? Nephrol Dial Transplant 2009; 24:1009‑1014. [ Links ]
9. Szeto CC, Chow KAM, Chung KY, Kwan BC, Li PK. Peritoneal protein and albumin excretion as markers of cardiovascular risk factor and systemic endothelial dysfunction. Hong Kong J Nephrol 2004; 6:31‑37. [ Links ]
10. Kathuria P, Moore HL, Khanna R, Tardowski ZJ, Goel S, Nolph KD. Effect of dialysis modality and membrane transport characteristics on dialysate protein losses of patients on peritoneal dialysis. Perit Dial Int 1997; 17:449‑454. [ Links ]
11. Churchil DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D. The Canada‑USA (CANUSA) Peritoneal Dialysis Study Group: Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. J Am Soc Nephrol 1998; 9:1285‑1292. [ Links ]
12. Balafa O, Halbesma N, Struijk DG, Dekker FW, Krediet RT. Peritoneal albumin and protein losses do not predict outcome in peritoneal dialysis patients. Clin J Am Soc Nephrol 2011; 6:561‑566. [ Links ]
13. Perez‑Fontan M, Rodriguez‑Carmona A, Barreda D, Lopez‑Muniz A, Blanco‑Castro N, Garcia‑Falcon T. Peritoneal protein transport during the baseline peritoneal equilibration test is an accurate predictor of the outcome of peritoneal dialysis patients. Nephron Clin Pract 2010; 116:104‑113. [ Links ]
14. Perl J, Huckvale K, Chelar M, John B, Davies SJ. Peritoneal protein clearence and not peritoneal membrane transport status predicts survival in a contemporary cohort of peritoneal dialysis patients. Clin J Am Soc Nephrol 2009; 4:1201‑1206. [ Links ]
15. Heaf JG, Sarac S, Afzal S. A high peritoneal large pore fluid flux causes hypoalbuminaemia and is a risk factor for death in peritoneal dialysis patients. Nephrol Dial Transplant 2005; 20: 2194‑2201. [ Links ]
16. Szeto CC, Chow KM, Lam CW, et al. Peritoneal albumin excretion is a strong predictor of cardiovascular events in peritoneal dialysis patients: a prospective cohort study. Perit Dial Int 2005; 25:445‑452. [ Links ]
17. Chow KM, Szeto CC, Law MC, Fun Fung JS, Kam–Tao Li P. Influence of peritoneal dialysis training nurses experience on peritonitis rates. Clin J Am Soc Nephrol 2007; 2:647‑652. [ Links ]
18. Kotsanas D, Polkinghorne KR, Korman TM, Atkins RC, Brown F. Risk factors for peritoneal dialysis‑related peritonitis: can we reduce the incidence and improve patient selection? Nephrology (Carlton) 2007; 12:239‑245. [ Links ]
19. Chow KM, Szeto CC, Leung CB, Kwan BC, Law MC, Li PK. A risk analysis of continuous ambulatory peritoneal dialysis‑related peritonitis. Perit Dial Int 2005; 25:374379. [ Links ]
20. Chen HC, Smith SJ, Tow B, Elias PM, Farese RV Jr. Leptin modulates the effects of acyl CoA: diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest 2002; 109:175‑181.
21. Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 2007; 117:2362‑2368. [ Links ]
22. Sritippayawan S, Chiangjong W, Semangoen T, Aiyasanon N, Jaetanawanitch P, Sinchaikul S, et al. Proteomic analysis of peritoneal dialysate fluid in patients with different types of peritoneal membranes. J Proteome Res 2007; 6:4356‑4362. [ Links ]
23. Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta‑analysis:
Peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 2006; 17:2591‑2598.
24. Heaf J. Peritoneal transport: getting more complicated. Nephrol Dial Transplant 2012; 27:4248‑4251. [ Links ]
25. Yoowannakul S, Harris LS, Davenport A. Peritoneal protein losses depend on more than just peritoneal dialysis modality and peritoneal membrane transporter status. Ther Apher Dial 2018; 22(2):171‑177. [ Links ]
Sara Daniela Rodrigues
Centro Hospitalar Vila Nova de Gaia e Espinho – Unidade 1,
Pavilhão Satélite – Serviço de Nefrologia
Rua Conceição Fernandes 1079
4434‑502, Vila Nova de Gaia, Portugal;
E‑mail: danisrodrigs@gmail.com
Disclosure of potential conflicts of interest: None declared
Received for publication: Oct 28, 2018
Accepted in revised form: Dec 18, 2018














