Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.4 Lisboa dez. 2018
CASE REPORT
Membranous nephropathy successfully treated with a Ponticelli regimen in a patient with HIV: do not assume that a well-known secondary cause is the real cause!
Meng C1,2, Pereira L1,2, Guedes L3, Nunes A1,2, Pereira P4, Frazão JM1,2, Pestana M1,2
1 Nephrology Department, Centro Hospitalar São João, Porto, Portugal.
2 Nephrology and Infectious diseases Group, INEB/I3S – Instituto de Investigação e Inovação em Saúde, Porto, Portugal.
3 Medicine Department, Centro Hospitalar Entre Douro e Vouga, Santa Maria da Feira, Portugal.
4 Pathology Department, Centro Hospitalar São João, Porto, Portugal.
ABSTRACT
Idiopathic membranous nephropathy with high-risk criteria for renal disease progression is considered an indication for immunosuppressive treatment. HIV infection has been associated with membranous nephropathy in a minority of patients.
A 44-year-old female diagnosed with HIV infection 11 years ago was referred for a nephrology consultation due to nephrotic syndrome. She presented with peripheral edema for 2 months and normal blood pressure.
Serum creatinine was 0.74 mg/dL, total cholesterol 490 mg/dL, albumin 2.0 g/dL; urinary examination revealed leukoerythrocyturia and 24h proteinuria was 4.5g. Renal ultrasound showed normal-sized kidneys with preserved corticomedullary differentiation.
Kidney biopsy showed thickening of the glomerular basal membrane and staining with Masson trichrome showed sub-epithelial humps. Immunofluorescence was negative except for IgA (+), C3c (+) and IgG (+). A diagnosis of membranous nephropathy was made. Secondary causes, such as neoplasic, infectious and autoimmune, were ruled out. Despite 6 months of conservative measures, proteinuria increased to 11 g/day. Since HIV viral load had been undetectable for several years, along with a CD4+ T cell count persistently above 400/mm3, a modified Ponticelli regimen was started: 3 pulses of methylprednisolone (1g/day), followed by 60 mg of prednisolone/day at months 1, 3 and 5, and cyclophosphamide 200mg/day at months 2, 4 and 6. At the end of the treatment, there was a partial response with proteinuria 3.94 g/day, albumin 3.2 g/dL, and creatinine 0.8 mg/dL. At 48 months of follow-up, the patient is asymptomatic, with creatinine 0.84 mg/dL and proteinuria 0.97 g/day.
Conclusion: Membranous nephropathy should be considered in the differential diagnosis in patients with HIV infection complicated by nephrotic syndrome even in the absence of other coinfections and comorbidities typically associated with membranous nephropathy. In patients with sustained negative viral loads and at high risk of progression to end-stage renal disease, in whom secondary causes have been excluded, immunosuppressive therapy might be considered.
Keywords: HIV, Membranous nephropathy, Ponticelli.
INTRODUCTION
Membranous nephropathy (MN) remains one of the most common causes of nephrotic syndrome in adults, the majority of cases being idiopathic or primary. In about one third of the cases, the disease is linked to infections, neoplasic and auto-immune disorders1,2.
Differentiation among primary and secondary causes has important prognostic and therapeutic implications, as the treatment in secondary causes focuses on treating the underlying cause. Pathologically, the disease is characterized by an accumulation of immune complexes in the subepithelial side of the glomerular basement membrane. Some biopsy findings might point to secondary forms of MN, like mesangial deposits which are present in less than 10% of cases of primary MN but are important to identify, as their presence favors a secondary form3,4. Discovery of phospholipase A2 receptor antibody and thrombospondin type 1 domain containing 7A might help the clinician differentiating primary from secondary causes, although the distinction is not always straightforward5,6.
MN has been reported in HIV-positive patients7,8, often associated with infections (namely HBV and HCV), and in some, the response parallels viral suppression following initiation of HAART (highly active antiretroviral therapy)9. In others, treatment of the co-existing infections leads to resolving proteinuria and remission questioning the causality between HIV infection and MN.
Immunosuppression is indicated in primary forms that do not respond despite conservative measures in high-risk patients10,11. This includes male gender, hypertension, low albumin, high serum creatinine and persistent nephrotic range proteinuria1,12, in whom progression to end-stage renal disease (ESRD) might occur in 10 years. In HIV patients, the benefits of immunosuppression must outweigh the increased risks of infection and malignancy associated with therapy in an already impaired compromised milieu, and the best therapeutic approach remains undefined.
CASE REPORT
A 44-year-old Caucasian woman was observed in a nephrology consultation due to nephrotic syndrome. She had complained of an abrupt onset bilateral leg edema for 2 months with no other associated symptoms.
Physical examination was significant for bilateral leg edema and overweight. Blood pressure was 126/80 mmHg, heart rate 80 bpm, respiratory rate 12 cpm and body mass index was 40 kg/m2.
Bloodwork showed hemoglobin 11.1 g/dL, albumin 2.0 g/dL, total cholesterol 490 mg/dL, low density lipoprotein-cholesterol 335 mg/dL, triglycerides 275 mg/dL, urea 41 mg/dL, creatinine 0.74 mg/dL. Urinalysis revealed hematuria and proteinuria, with 24-hour proteinuria quantification of 11 grams. Complement, ANA, anti-ds-DNA, ANCA, anti-GBM, cryoglobulin, serum protein electrophoresis, and serum immunofixation were normal. With the exception of HIV, viral serologies were negative. Renal ultrasound showed normal-sized kidneys with normal corticomedullary differentiation.
Her medical history was significant for acquired immunodeficiency syndrome (AIDS) diagnosed 11 years before, disseminated tuberculosis being the AIDSdefining disease. Her current antiviral treatment consisted of raltegravir/abacavir/lamivudine for 5 years, with excellent adhesion, negative viral load and CD4 cell count persistently above 400 cells/μL.
Other relevant comorbidities included obesity and recurrent urinary tract infections (UTI) under prophylaxis with trimethoprim-sulfamethoxazole, which was started prophylactically upon the modified Ponticelli regimen for a period of 6 months, and resumed 8 months later as prophylaxis due to the recurrent UTI.
Renal biopsy revealed renal cortex with 12 glomeruli, none sclerotic. There was slight mesangial proliferation, with diffuse thickening of glomerular basement membrane (Figures 1 and 2). In addition, no significant fibrosis and tubular atrophy was described. Staining with Masson trichrome showed sub-epithelial humps.

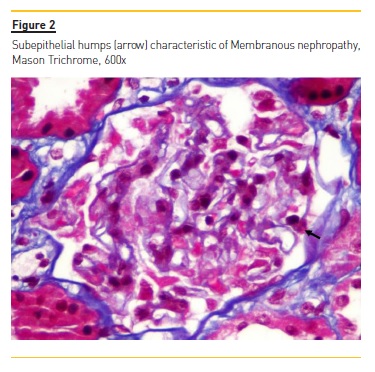
Immunofluorescence revealed only slight positivity (one +) for IgA, C3c and IgG, and was negative for C1q and C4. A diagnosis of MN was made. Unfortunately, electronic microscopy was not performed to assess the presence of other locations of the deposits, namely subendothelial.
A workup was performed in order to exclude other secondary causes of MN. This included mammography, cervical cytology, chest radiography, cervical, abdominal and gynecological ultrasound, upper endoscopy and colonoscopy as well as thyroid function. Serology for syphilis (VDRL and TTPA) was negative. IGRA (Interferon Gamma Release Assay) was not performed. This extensive study was normal. Unfortunately, at the time of the nephrotic presentation of this patient, our hospital did not have testing available for anti-phospholipase A2 receptor (PLA2R) autoantibody.
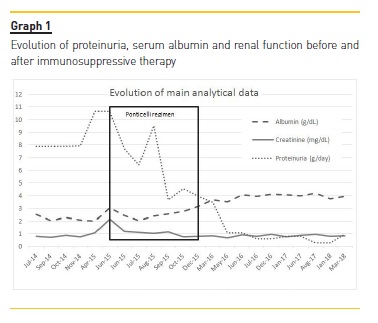
The patient was treated with furosemide, lisinopril (up to the maximum tolerated dose), atorvastatin and ezetimibe. Due to the possibility of improvement without immunosuppression, the patient was followed for 6 months but 24h proteinuria increased to 11g and renal function started to deteriorate (Graph 1).
Given the controlled HIV infection with years of evolution, the abrupt onset of nephrotic syndrome and exclusion of other known secondary causes, and as patient remained with proteinuria above 8 grams/day and with slight decline of renal function, immunosuppression was considered. After discussing with patient and her infectious disease specialist, a modified Ponticelli regimen was initiated – 3 pulses of methylprednisolone 1 g/day followed by prednisolone 60 mg/day in months 1, 3 and 5; cyclophosphamide 200 mg/day (2mg/kg/day) in months 2, 4 and 6.
At 5 months of immunosuppressive treatment, the patient had mild genital herpes due to herpes virus 2 and was treated with valaciclovir, without need for reduction/suspension of immunosuppression. This was the only infectious intercurrence during the treatment.
During the Ponticelli regimen and in the follow-up period, leg edema resolved, proteinuria gradually decreased, renal function normalized and the patient reported no other infectious complications. Evolution of proteinuria and renal function is shown in Graph 1. At 48 months of follow up, the patient is asymptomatic, with creatinine 0.84 mg/dL and proteinuria 0.97 g/day.
DISCUSSION
MN constitutes the most common cause of nephrotic syndrome in Caucasian adults13. As Idiopathic and secondary forms share the same clinical features, distinction between both forms is crucial. Idiopathic/primary MN (iMN) is diagnosed after excluding all secondary causes for treatment purposes. Some histological patterns are suggestive of iMN, such as an exclusive subepithelial location of the deposits, while subepithelial, intramembranous, and mesangial deposits suggest secondary forms1. IgG subclass staining may further help to classify MN. IgG1, IgG2, and IgG3 generally dominate in the deposits of secondary MN, whereas a preponderance of IgG4 is characteristic for primary MN3. Unfortunately, neither IgG subtype staining nor EM was performed in our sample, which could have been useful to differentiate between primary and secondary forms of MN.
The classic renal lesion in patients with HIV is the so-called HIV-associated nephropathy (HIVAN), which is characterized by a triad of collapsing glomerulopathy, microcystic tubular dilatation, and endothelial cell tubuloreticular inclusions. However, other histological patterns have been described as the incidence of HIVAN decreases. MN has been described in HIV positive patients in case reports8,14-16 and case series9,17-20.
Some of these patients9,15 were HBV, HCV negative, with high viral loads of HIV RNA, and experienced remission following viral suppression after HAART therapy.
Our patient had sustained negative RNA measurements and CD4 counts above 400 cells/mm3, which does not favor a causal relationship between HIV and renal disease.
However, most of these patients had simultaneously other diseases that are often associated with secondary causes of MN (HBV infection, syphilis, SLE), therefore making it difficult to establish the independent contribution of HIV to these histopathological changes. To support the difficulty of establishing a causal association between MN and HIV infection, in a South African cohort of 99 HIV-positive patients, the prevalence of MN was 13%, but almost a third of these were either HBV or HCV positive, and the prevalence did not differ amongst a HIV-negative control group with nephrotic syndrome21.
Immunodeficiency and dysregulation of immunoglobulin synthetic responses and T-cell function and large amounts of circulating antigen are associated with a polyclonal antibody response. This could lead to the formation of immune complexes, either in the circulation or in situ in the kidney itself, producing histological patterns such as lupus-like and MN, now designated as part of HIV immune complex disease of the kidney (HIVICK)22. However, the absence of a complete response using treatment focused entirely on suppressing the production of such immune complexes through suppression of viral replication using antiretroviral therapy, and its occurrence in patients with negative viral load, such as in our patient, suggests that another mechanisms contribute to the pathophysiology of MN in these patients17.
M-type phospholipase A2 receptor (PLA2R), which can be found in approximately 70% of iMN patients, might represent a specific marker of iMN and also correlate with disease activity and prognosis5. Unfortunately, the assay was not available at the time of diagnosis, and the accuracy of PLA2R antigen essay to distinguish primary from secondary membranous nephropathy has not yet been established in the context of HIV19. In HBV patients, the positivity of PLA2R might be due to induction of PLA2R autoimmunization23,24.
The more recently discovered antibody against the Thrombospondin type-1 domain-containing 7A (THSD7A)25 might also be useful in distinguishing primary from secondary causes, since it represents 10-16% of PLA2R-negative cases26. However, its expression has also been associated with an increased risk of malignancy. Hoxla et al27 report an association between THSD7A-positive MN and gallbladder cancer, with disappearance of the antibody following chemotherapy, proposing that tumor cells express the THSD7A28. Therefore, more studies are needed to establish the role of anti-THSD7A in clinical practice.
The clinical course of MN varies with up to one third of patients experiencing spontaneous remission4. The other two-thirds might maintain proteinuria or experience progression to ESRD. Patients at high risk of progression present hypertension, low albumin, high serum creatinine and persistent nephrotic range proteinuria11.
The presence of nephrotic range proteinuria increases the risk of progression to ESRD four-fold4 with progression to ESRD over 10 years, notwithstanding the complications associated with nephrotic syndrome (infections, thromboembolic events, and accelerated atherosclerotic cardiovascular disease) and these patients are best managed using immunosuppressive therapy10.
Concerns over immunosuppressive agents in patients with HIV infection are legitimate, such as the fear of precipitating opportunistic infections. Steroids have been part of the treatment regimen of infections such as Pneumocystis jirovecci in HIV patients and have been used in anecdotical reports with proteinuria remission.
El-Husseini et al7 have used adrenocorticotropic hormone in a patient, achieving remission. No data are available regarding the use of alkylating agents in this population, although they remain the highest-level evidence for treatment for high-risk patients. While cyclophosphamide has been associated with na increased risk of viral, fungal, parasitic and bacterial infections, including tuberculosis, no recommendations regarding tuberculosis screening in HIV patients prior to the institution of cyclophosphamide have been published, unlike with drugs such as rituximab or anti-TNFα agents. IGRA testing performs poorly in HIV patients, with a meta-analysis showing a pooled sensitivity of only 61% in culture-proven tuberculosis29. Nevertheless, its use has been recommended in current guidelines30,31.
As our patient had a prior history of tuberculosis, we acknowledge that screening for latent tuberculosis might have been useful due to the increased risk in the setting of use of immunosuppressive therapy. Rituximab has been proposed as a safe alternative, with observational studies with variable protocols32,33 showing promising results with few adverse events and one RCT34 showing a statistically significant remission rate compared to placebo 6 months after therapy. Ongoing trials, comparing the efficacy of rituximab with cyclosporine35 and sequential therapy with tacrolimus and rituximab versus the modified Ponticelli regimen36, are expected to be completed later this year, clarifying the role of the drug as a firstline agent for iMN. The long-term effects of B-cell depletion are unknown, and the use of rituximab in the HIV population is limited, mostly as an adjuvant drug in HIV-related lymphomas in which it appears to be safe in selected patients37,38, although these results could not be replicated in a randomized controlled trial39.
Further studies are needed to establish the safety of rituximab in HIV patients with other malignancies and non-malignant diseases.
In our case, the absence of active infection, a negative work-up for secondary causes, and a persistent negative viral load with high CD4 cell counts, coupled with a young patient at high risk of progression to ESRD, prompted institution of therapy for high-risk iMN, using the modified Ponticelli regimen with a close follow-up. To our knowledge, this is the first published case describing the use of this protocol in HIV patients.
CONCLUSION
Membranous nephropathy should be considered in the differential diagnosis in patients with HIV infection complicated by nephrotic syndrome even in the absence of other coinfections and comorbidities typically associated with MN. The role of PLA2R remains to be determined in this subset of patients. Treatment options must weigh up the risk/benefit ratio, and in patients with sustained negative viral loads and in high risk of progression to ESRD, in whom secondary causes have been excluded, immunosuppressive therapy might be considered.
References
1. Fervenza FC, Sethi S, Specks U. Idiopathic Membranous nephropathy: Diagnosis and treatment. Clin J Am Soc Nephrol. 2008;3(3):905-19. [ Links ]
2. Hofstra JM, Fervenza FC, Wetzels JFM. Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol. 2013;9(8):443-58. [ Links ]
3. Gameiro J, Jorge S, Lopes JA, Correia L, Gomes da Costa A. Membranous nephropathy: A diagnostic and therapeutic challenge? Port J Nephrol Hypert. 2017;31:42-6. [ Links ]
4. Cattran DC, Brenchley PE. Membranous nephropathy: integrating basic science into improved clinical management. Kidney Int. 2017;91(3):566-74. [ Links ]
5. Bomback AS. Management of membranous nephropathy in the PLA2R Era. Clin J Am Soc Nephrol. 2018;13(5):784-6. [ Links ]
6. De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC. A Proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28(2):421-30. [ Links ]
7. El-Husseini A, Saxon D, Jennings S, Cornea V, Beck L, Sawaya BP. Idiopathic membranous nephropathy: diagnostic and therapeutic challenges. Am J Nephrol. 2016;43(2):65-70. [ Links ]
8. Numata A, Akimoto T, Toshima M, Iwazu Y, Otani N, Miki T, et al. Membranous nephropathy in an HIV-positive patient complicated with hepatitis B virus infection. Clin Exp Nephrol. 2011;15(5):769-73. [ Links ]
9. Aydin S, Mete B, Yilmaz M, Yenidünya G, Zaras R, Tunckale A, et al. A patient with HIV infection presenting with diffuse membranous glomerulonephritis in a country with a low HIV prevalenceRemarkable remission with therapy. J Infect Public Health. 2012;5(2):207-10. [ Links ]
10. Cattran DC, Feehally J, Cook HT, Liu ZH, Fervenza FC, Mezzano SA, Wetzels JFM. Kidney disease: Improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2(2):139-274. [ Links ]
11. Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12(6): 983-97. [ Links ]
12. Cattran D. Management of membranous nephropathy: when and what for treatment. J Am Soc Nephrol. 2005;16(5):1188. [ Links ]
13. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases: Elsevier Health Sciences; 2017. [ Links ]
14. Paueksakon P, Grewal M, Shappell S. Nephrotic range proteinuria and hematuria in a white bisexual male. Am J Kidney Dis. 1999;33(3):607-12. [ Links ]
15. Alarcon‐Zurita A, Salas A, Antón E, Morey A, Munar MA, Losada P, et al. Membranous glomerulonephritis with nephrotic syndrome in a HIV positive patientremarkable remission with triple therapy. Nephrol Dial Transplant. 2000;15(7):1097-8. [ Links ]
16. López-López L, González A, Vilá LM. Long-term membranous glomerulonephritis as the presenting manifestation of systemic lupus erythematosus in a patient with human immunodeficiency virus infection. Lupus. 2012;21(8):900-4. [ Links ]
17. Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-52. [ Links ]
18. Han TM, Naicker S, Ramdial PK, Assounga AG. A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int. 2006;69(12):2243-50. [ Links ]
19. Booth JW, Hamzah L, Jose S, Horsfield C, ODonnell P, McAdoo S, et al. Clinical characteristics and outcomes of HIV-associated immune complex kidney disease. Nephrol Dial Transplant. 2016;31(12):2099-107. [ Links ]
20. Foy MC, Estrella MM, Lucas GM, Tahir F, Fine DM, Moore RD, et al. Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clin J Am Soc Nephrol. 2013;8(9):1524-32. [ Links ]
21. Gerntholtz TE, Goetsch SJW, Katz I. HIV-related nephropathy: A South African perspective. Kidney Int. 2006;69(10):1885-91. [ Links ]
22. Nobakht E, Cohen SD, Rosenberg AZ, Kimmel PL. HIV-associated immune complex kidney disease. Nat Rev Nephrol. 2016;12(5):291-300. [ Links ]
23. Berchtold L, Zanetta G, Dahan K, Mihout F, Peltier J, Guerrot D, et al. Efficacy and safety of rituximab in hepatitis B virus–associated PLA2R-positive membranous nephropathy. Kidney Int Rep. 2018;3(2):486-91. [ Links ]
24. Xie Q, Li Y, Xue J, Xiong Z, Wang L, Sun Z, et al. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol. 2015;41(4-5):345-53. [ Links ]
25. Tomas NM, Beck LH, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371(24):2277-87. [ Links ]
26. Wang J, Cui Z, Lu J, Probst C, Zhang YM, Wang X, et al. Circulating antibodies against thrombospondin type-I domain-containing 7A in chinese patients with idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2017;12(10):1642-51. [ Links ]
27. Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, et al. A mechanism for cancer-associated membranous nephropathy. N Engl J Med. 2016;374(20):1995-6. [ Links ]
28. Sharma SG, Larsen CP. Tissue staining for THSD7A in glomeruli correlates with serum antibodies in primary membranous nephropathy: a clinicopathological study. Mod Pathol. 2018;31(4):616-22. [ Links ]
29. Santin M, Muñoz L, Rigau D. Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and metaanalysis. PLoS ONE. 2012;7(3):e32482-e. [ Links ]
30. National Institute for Health and Care Excellence (2016) Tuberculosis (NICE Guideline 33). 2016 [Available from: Available at: https://www.nice.org.uk/guidance/ng33/resources/tuberculosis-1837390683589. [ Links ]
31. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection-United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25. [ Links ]
32. Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23(8):1416-25. [ Links ]
33. Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73(1):117-25. [ Links ]
34. Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, et al. Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up. J Am Soc Nephrol. 2017;28(1):348-58. [ Links ]
35. Fervenza FC, Canetta PA, Barbour SJ, Lafayette RA, Rovin BH, Aslam N, et al. A Multicenter Randomized Controlled Trial of Rituximab versus Cyclosporine in the Treatment of Idiopathic Membranous Nephropathy (MENTOR). Nephron. 2015;130(3):159-68. [ Links ]
36. Rojas-Rivera J, Fernandez-Juarez G, Ortiz A, Hofstra J, Gesualdo L, Tesar V, et al. A European multicentre and open-label controlled randomized trial to evaluate the efficacy of Sequential treatment with TAcrolimus-Rituximab versus steroids plus cyclophosphamide in patients with primary MEmbranous Nephropathy: the STARMEN study. Clin Kidney J. 2015;8(5):503-10. [ Links ]
37. Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119(14):3245-55. [ Links ]
38. Wyen C, Jensen B, Hentrich M, Siehl J, Sabranski M, Esser S, et al. Treatment of AIDSrelated lymphomas: rituximab is beneficial even in severely immunosuppressed patients. AIDS. 2012;26(4):457-64. [ Links ]
39. Kaplan LD, Lee JY, Ambinder RF, Sparano JA, Cesarman E, Chadburn A, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDSMalignancies Consortium Trial 010. Blood. 2005;106(5):1538-43. [ Links ]
Luciano Artur Lopes Pereira;
Nephrology Department, São João Hospital Center, Alameda Prof.
Hernani Monteiro, 4200-319 Porto.
E-mail: lucianoarturpereira@hotmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: Aug 26, 2018
Accepted in revised form: Nov 20, 2018














