Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.33 no.1 Lisboa mar. 2019
https://doi.org/10.32932/pjnh.2019.04.008
REVIEW ARTICLE
Current guidelines in peritoneal dialysis – Part II
Ana Carina Ferreira1,2, Andreia Borges3,4, Cristina Pinto Abreu5, Fernando Teixeira e Costa6, Marta Pereira5, Rui Castro7, Sara Barreto6, on Behalf of the Peritoneal Dialysis Study Group of the Portuguese Society of Nephrology (annex 1)
Peritoneal Dialysis Study Group of the Portuguese Society of Nephrology (annex 1)
1 Department of Nephrology of Curry Cabral Hospital – Centro Hospitalar e Universitário de Lisboa Central, Lisbon, Portugal
2 Nova Medical School, Faculdade de Ciências Médicas, Universidade Nova de Lisboa, Lisbon, Portugal
3 Department of Nephrology, Centro Hospital Universitário de Coimbra, Coimbra, Portugal
4 University Clinic of Nephrology, Faculty of Medicine, Universidade de Coimbra, Coimbra, Portugal
5 Department of Nephrology of Centro Hospitalar de Lisboa Norte, Lisbon, Portugal
6 Department of Nephrology of Hospital Gracia de Orta, Almada, Portugal
7 Department of Nephrology of Centro Hospitalar de Trás-os-Montes e Alto Douro, Vila Real, Portugal
ABSTRACT
A successful peritoneal dialysis program depends on knowledge of the current recommendations and on evidence-based practice guidelines. In this second article, we review the dialysis prescription and the nutritional and cardiovascular management of peritoneal dialysis patients in the light of the existing guidelines advocated by different international societies.
Key words: peritoneal dialysis, guidelines, dialysis dose, nutrition, cardiovascular disease;
INTRODUCTION
Peritoneal dialysis (PD) is well-established dialysis technique used to treat uremic patients since 1970s. Although underused, this modality has many advantages, namely preservation of renal residual function (RRF), hemodynamic stability, the maintenance of quality of life and cost savings1, among others. A successful PD program is dependent on many variables, and a good training of both physicians and PD nurses is essential. Also, the knowledge of current recommendations, centered on evidence-based practice guidance is of extreme importance.
This is part II of a review article about the current existing guidelines in adult patients and focuses on dialysis prescription and metabolic and cardiovascular management and nutrition. Part I reviewed the current guidelines on catheter insertion and catheter-related infections. For the purpose of these two articles, we looked at the International Society of Peritoneal Dialysis (ISPD) guidelines, the UK Renal Association guidelines, European Renal Best Practices (ERBP), Kidney Disease Improving Global Outcomes (KDIGO), and the Portuguese Good Practice Manual in Chronic Dialysis.
CURRENT GUIDELINES ON PERITONEAL DIALYSIS
Guidelines on Efficacy – fluids and solutes removal
Fluids removal
Euvolemia is a predictor of outcome in PD patients as volume overload is related to cardiac dysfunction2,3 inflammation4 and mortality5.
Fluid overload is indeed a prevalent problem in peritoneal dialysis patients ranging from 22% to 72% in different studies6-9 and more attention should be given to its assessment and correction.
Recent focus on PD adequacy has moved from small solute clearance to fluid removal and preservation of residual function. Observational studies have shown a strong and consistent association between higher levels of RRF and improved patient survival on PD10,11.
Adequacy of peritoneal dialysis in Mexico (ADEMEX) showed no survival advantage to an increased dose of small-molecule clearance delivered by PD, but found an association of fluid overload and mortality10.
A reanalysis of the large CANUSA study has shown that decreased RRF, rather than peritoneal creatinine clearance, best predicts both mortality and morbidity in PD patients11. According to this analysis, every increase of 250 ml in urine output leads to a 36% decrease in mortality risk, suggesting the important role of fluid status in predicting clinical outcome. Associations between peritoneal fluid removal and survival have been found in a study from Turkey into
CAPD patients12. In that study, Ates et al found that total fluid removal, including ultrafiltration (UF) and urine output, was an independent predictor of patient survival. The European APD Outcome Study (EAPOS) found that a baseline ultrafiltration less than 750 ml in 24h predicted a poor survival in anuric automated PD patient13. Additionally, the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) also show that low ultrafiltration in anuric patients on PD is associated with a decreased survival14. Another study performed in a single center in a Chinese population demonstrated a strong predictive value of daily peritoneal ultrafiltration for both technique and patient survival in anuric PD patients15.
1. Fluid removal and regulation of volume
Fluid overload in PD patients is often multifactorial. Fluid status is a balance between fluid output (UF and/or diuresis) and fluid input (fluid and salt intake). So, despite good UF and residual diuresis, patients still can have fluid overload, stressing the important role of dietary restriction of salt and fluid intake. Adherence to a sodiumrestricted diet is critical, and dietary education should be a part of each patients care plan.
The discrepancy between ultrafiltration and fluid overload makes it very difficult to formulate UF targets in guidelines for adequacy of PD. Nevertheless this has been tried. The ISPD defines UF failure (UFF) as net UF less than 400 ml after a dwell of 4 h with 4% glucose based dialysis solution (3x4 definition). This definition is useful because it distinguishes peritoneal UF failure from fluid overload16.
In patients with RRF, it is difficult to set a fixed UF volume. In these patients, maintaining a clinical state without edema, hypertension and cardiac overload is most important than achieving a particular level of UF volume. The ISPD guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis does not include specific suggestions other than the statement ”Attention should be paid to both urine volume and the amount of UF, with the goal of maintaining euvolemia”17.
Other societies have provided further recommendations. The Committee of European Best Practice Guidelines decided to formulate targets based on those achieved by dialysis only: ”these targets should be adequate for anuric PD patients; the presence of RRF compensate in situations where the peritoneal targets are not achieved”. They decided on an arbitrary target of 1L/day for net UF in anuric patients18.
The Canadian Society of Nephrology Clinical Practice Guidelines and Recommendations on PD adequacy19 suggests that a low net daily peritoneal UF volume (< 750 ml in anuric or <250 ml in patients with RRF) be an indication for careful evaluation of volume status (looking for evidence of fluid overload) and of dietary fluid and fluid intake (looking for evidence of insufficient intake or malnutrition). The Renal Association Clinical Practice Guideline on PD in adults and children20 recommend that anuric patients who consistently achieve a daily UF of less than 750 ml should be closely monitored.
One note of importance is the potential impact of bag overfills on the overestimation of UF and fluid overload21. It can be that the overestimation of real UF by neglecting overfill can lead to overhydration, as it gives the patient and physician the false feeling of adequate UF.
2. Assessment of volume status
All PD patients should undergo regular assessment of volume status. The frequency of assessment is determined by the clinical stability of the patient, but should occur at least every 1-3 months. UF is important but not the only factor that can lead to overhydration.
Mismatches between fluid intake, urine production and peritoneal fluid removal are common causes of overhydration. Peritoneal membrane failure should be diagnosed only after consideration of other causes of fluid overload and after a PET test. Mechanical cause such as peritoneal leak or catheter dysfunction should be ruled out16.
Fluid status in PD patients can be assessed in different ways and the prevalence of fluid overload depends on which method is used(22-26). Clinical observation is mandatory, but alone is insufficient to correctly identify minor deviations from normohydration. It has a high specificity but a low sensitivity for overhydratation22. Serum markers such as BNP reflect a mix of cardiac dysfunction and circulating volume overload. In PD patients, proof of its clinical usefulness is limited23.
Lung ultrasound (US) can be used to assess the extravascular water content of the lung and has been used in PD. Based on chest US moderate to severe lung congestion was detected in a significant proportion (46%) of asymptomatic PD patients24. Bioimpedance analysis (BIA) is an easy to perform non-invasive technique and should be performed according to a standardized procedure. It has been linked to mortality in PD patients25. BIA has a good reproducibility and longitudinal studies show that BIA is a good tool for identifying changes in fluid status that otherwise might not be clinically identifiable. Luo et al showed that BIA could help guide fluid control in PD patients26.
The EuroBCM trial6 revealed that only 40% of the patients enrolled were euvolemic; severe fluid overload was present in 25.2% and that blood pressure and volume status had a poor correlation. Moreover, hydration status was comparable in APD and CAPD patients. Nevertheless, it should be taken into account that the measures of fluid overload differ among studies and have no direct relationship to circulating volume. Aggressive ultrafiltration based on a single measurement is not recommended. Both congestion and dehydration threaten RRF.
Only careful evaluation of the trend of BIA with clinical judgment should dictate prescription changes. Another important fact is that body composition such as malnutrition and obesity changes fluid redistribution. In fact body cell mass (nutrition) is related to intracellular water (ICW); fat mass is associated with extracellular water (ECW); the ratio ECW/TBW (total body water) increased with overhydration, but also increases when ICW decreases (malnutrition) and when fat mass increases (obesity). Therefore, these issues should be considered when applying BIA derived fluid overload measurements.
3. Assessment of peritoneal membrane function
The functional assessment of the peritoneal membrane, specifically solute transport rate and ultrafiltration capacity, provides useful information on the correct prescription of the peritoneal dialysis regime and it makes it possible to monitor changes in peritoneal membrane over time.
The majority of guidelines18-20,27-30 recommended performing a baseline peritoneal kinetic analysis 4–8 weeks and no more than 3 months after starting PD in order to provide another useful tool in the process of prescribing an adequate dialysis regimen and to evaluate the initial characteristics of the peritoneum. No clear consensus exists for repeating the evaluation at different points. Some guidelines27 recommended not repeating the PET at preset intervals, rather to repeat the test when clinical problems arise. Other guidelines16 suggest performing the evaluation at least once a year and whenever clinically indicated.
We think that peritoneal kinetics must be performed every year as a standard practice and/or when clinical problems arise such as volume overload or insufficient dialysis and following cases of peritonitis, above all if the episode is aggressive (1 month after resolution) or if a decrease in UF is detected. In addition, a recent PET test should be available before the change from CAPD to APD. Frequent mistakesare made in prescribing APD without knowledge of membrane small solute transport rate or water removal capacity. Slow transporters are better managed with CAPD while fast transporters are better managed with APD. The concept of “adapted PD” indeed focuses on membrane physiology towards a better prescription. Without a previous PET test, dwell times and volumes might be wrongly prescribed. The measurement of intraperitoneal pressure is a tool that can also help individualize intraperitoneal volume prescription in order to optimize adequacy while avoiding counteracting UF or promoting hernias.
There is insufficient evidence to prefer one test of peritoneal membrane characteristic to another for clinical prescription20. We prefer modified PET (3.86% instead 2.27%) with temporary drainage after 1 hour for weighing and sampling, followed by reinfusion and final drainage after 4 hours for several orders of reasons:
– This methodology is recommended by the ISPD in order to define UF failure (<400 ml) and to obtain standardize measure of UF;
– The peritoneal membranes UF capacity is easier to quantify because of the higher quantity of UF that can be obtained; the coefficient of variation of UF has been quantified at approximately 50% for the 2.27% PET and < 10 % for the 3.86% PET31;
– The sodium sieving value at 60 minutes allows for the examination of aquaporin function;
– The results obtained in terms of D/P creatinine ratios are compared to those obtained using a PET 2.27% which allow the continued classification of patients based on their capacity for small molecules transport;
– Is better at differentiating between small pore UF and free water transport (UF across the aquaporin-1 channels).
Reduced or absent free water transport may contribute to reduced UF capacity or UF failure, as it represents approximately 50% of peritoneal UF in the first part of an exchange with a hypertonic solution. The loss of free water transport and the osmotic conductance to glucose should suggest severe alterations in peritoneal membrane32.
The double mini-PET measures the osmotic conductance to glucose i.e., the capacity to generate UF with the osmotic stimulus of glucose that is more or less hypertonic. It indicates the amount of UF that can be obtained by increasing of glucose in PD solution. Indeed osmotic conductance measures the capacity to generate UF by both pores and, therefore, a reduction indicates a decreased in the peritoneums overall capacity to generate UF33. In cases of UFF, the double mini-PET provides additional indications for the prescription of the most suitable PD mode.
4. Avoidance of routine utilization of hypertonic glucose solutions
Peritoneal dialysis UF becomes increasingly important as RRF declines and can be modified by adjusting the type of PD solutions use. Increasing the strength of glucose solution can enhance UF volume by increasing the osmotic gradient. However, there is the risk of enhanced fluid intake, not only because of hyperglycemia associated with poor glycemic control, but also because of hypernatremia associated with sodium sieving enhances thirst. The regular use of hypertonic glucose dialysis fluid is associated with systemic effects such as weight gain34, poor diabetic control, delayed gastric empting35 and furthermore it is associated with a more rapid deterioration of peritoneal membrane function36,37.
As RRF decreases, icodextrin can and should be added to enhance UF volume. The removed volume using icodextrin will also eliminate more sodium than the same UF volume when using glucose based solutions. Compared with the use of 4.25% glucose, use of icodextrin increases UF volumes in long duration dwells38,39 and leads to a sustained reduction in extracellular fluid volume40,41. In the Cochrane review by Cho et al, prescription of icodextrin was associated with improved peritoneal UF and mitigated uncontrolled fluid overload42.
Icodextrin is recommended for dwells longer than 8h such as in the day dwell APD and the overnight dwell in CAPD. There is preliminary evidence that twice daily icodextrin may be safe and may enhance UF in patients with evidence of UFF43,44.
5. Adaptation of PD protocols to the peritoneal membrane transport
The concept that fast transport status is linked to overhydration is well established in the minds of many45-47. A meta-analysis48 of a number of prospective observational studies confirmed a worse prognosis (particularly in terms of survival) for high transporters than for patients with lower or slower transport characteristics. The same study demonstrated that treatment with APD, in a subgroup of patients, made the peritoneal transport characteristic non-influential in terms of patient survival. It is, then, important to keep the message that selective APD prescription, particularly taking into account peritoneal membrane transport status, has changed the outcome of fast transporters.
A fast transporter status is no longer a threat if adequately managed with updated prescription.
Fast transporters have a rapid dissipation of their glucose gradient, and thus negative UF during longer dwells. Therefore, for fast transporters, short dwells are recommended29, and APD with its shorts dwells might lead to better outcomes for these patients49. Some authors50 have reported that with the advent of APD and icodextrin, the mortality of fast transporters, which in the past was much higher than for other transporter types, has become similar to that of patients belonging to other transporter categories. The use of icodextrin for the long exchange will prevent fluid reabsorption.
For slow transporters, manual CAPD is indicated, with high single exchange volumes29. Too short dwells can induce sodium sieving in slow transporters and thus lead to removal of free water but not of sodium, resulting in fluid retention51,52. But if APD is performed with a low number of cycles, avoiding too short dwell times and sodium sieving, there are no differences in sodium removal or volume control between APD and CAPD.
6. Preservation of RRF
As mentioned above, RRF is one of the most important factors that predict survival in PD patients and also helps maintain extracellular volume.
The EURO-BCM study showed that there is a tendency toward development of overhydration in patients with reduced renal diuresis6.
Similarly in the study by Konings et al53 a statistically significant relationship between state of hydration and RRF was found: PD patients with RRF less than 2 ml/min had increased levels of extracellular fluid compared with those who had better preserved residual GFR. Unfortunately, RRF declines during treatment by PD. Longitudinal studies have shown that RRF declines progressively with time on dialysis. In general, most PD patients will have lost their RRF within 3-5 years after the start of their treatment54. In the CANUSA study11, RRF decreased from 3.8 to 1.4 ml/ min over a mean follow-up period of 2 years.
Preservation of RRF plays a central role for achieving adequacy of dialysis. Adequate strategies to preserve RRF should be pursued. These include the use of ACEi, ARBs, diuretics, and avoidance of nephrotoxic drugs and hypovolemia. ACE inhibitors (ramipril 5 mg) and ARBs (valsatan) have been reported in two RCT to be effective for the preservation of RRF in patients undergoing PD55,56. Recently, Zhang et al57 systemically reviewed the effect of ACEI and ARB in preserving RRF in PD patients, and found that blocking the renin-angiotensin-aldosterone system (RAAS) with ACEi or ARBs may halt the decline in RRF in PD patients.
The use of high dose diuretics in individuals with RRF is supported by a RCT conducted in incident CAPD patients who received either furosemide 250 mg daily (plus metalazone 5 mg daily if diuresis was less than 500 ml in 24h) or no diuretics and were followed for 12 months. Patients treated with diuretics experienced an increase in urine output and urinary sodium excretion with no difference in the rate of loss of RRF compared with the control group58.
The greatest challenge in the management of PD patients consists of removing the correct amount of fluid, avoiding both fluid excess and fluid depletion. It has been argued that patients should be keptslightly overhydrated to preserve RRF because this has been associated with improved outcomes. However, retrospective observational data seem to indicate that an increased ECW/TBW ratio is not associated with preservation of RRF59,60.
Episodes of volume depletion are associated with increased risk of loss in RRF61. Dehydration can cause acute kidney injury (AKI) and loss of RRF; this may be attributed to the functional reductions of aquaporins, renal vasoconstriction and structural changes in tubular system in the condition of hypovolemia62. Differing results have been found regarding the use of aminoglycosides and their effect on RRF63,64. When alternative acceptable antibiotics are available, aminoglycosides should be avoided in the setting of preserved RRF. Avoidance of other nephrotoxic drugs such as non-steroidal anti-inflammatory medications and intravenous contrast dye may also be beneficial in preserving RRF65,66.
Traditional PD solutions are rich in glucose degradation products (GDPs), which have been demonstrated to be associated with higher serum levels of advanced glycation end products and progressive renal injury67. Modifying the peritoneal dialysate by raising pH, reducingglucose, and using non-lactate fluids as a buffer was thought to lessen the adverse effects of conventional PD solutions. Previous small, randomized controlled trials (RCTs) have demonstrated conflicting results with respect to the effects of biocompatible PD solutions on RRF decline in PD patients compared with conventional PD solutions68-72. Yohanna et al73 systematically reviewed 11 trials in which 643 patients were included. They reported that the use of a neutral-pH, low-GDP solution resulted in better preserved RRF after various study periods.
In the balANZ trial, the largest and highest quality RCT of biocompatible solutions examining RRF published to date, the primary unadjusted analysis found a non-significant (p = 0.06) difference in RRF decline between the intervention and control groups across the first and second 12 months of the study. Nonetheless, the biocompatible group did experience a significantly lower hazard of anuria (hazard ratio [HR] 0.36; 95% confidence interval [CI], 0.13 – 0.96)74. In the secondary analysis of the balANZ trial75, after adjusting for a range of demographic, clinical and dialysis characteristics, the use of biocompatible PD solution was associated with 27% better preservation of RRF (p = 0.004) and 37% better preservation of residual urine volume (p < 0.001) compared with the use of conventional PD solution. These findings are in keeping with the consistent findings of systematic reviews and meta-analyses of RCTs those biocompatible PD solutions better preserve RRF and residual urine volume compared with conventional solutions42,73,76,77. The results of this study therefore further support these systematic reviews and the recommendations of the International Society for Peritoneal Dialysis (ISPD) guidelines that neutral pH, low glucose degradation product PD solutions should be used for better preservation of RRF78.
Solutes removal
Solutes removal guidelines for peritoneal dialysis (PD) clinicians are comparable to a beacon for a sailor. To reach optimal results, they need a sense of orientation regarding their objectives, well being of their patients and reaching their seaport, respectively.
We reviewed the ISPD guidelines17, the UK Renal Association guidelines (RA)20, ERBP18, KDOQI79, Canadian Society of Nephrology (CSN) Guidelines/ Recommendations19 and also the Portuguese Good Practice Manual (PGPM) in Chronic Dialysis solutes clearance recommendations80 (Table 1).
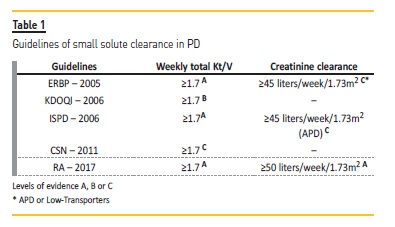
The weekly Kt/V is a standard index of PD solute removal. Several guidelines reinforce it as a reproducible and practical method to reach adequate dialysis dose for PD patients. Nevertheless, some authors disputed this consensus, with reasonable scientific basis, and presented new solute clearance markers, like the Wang L. dialysis index (DI), which includes the dietary intake in the clearance rate81.
The calculation of V (volume) is a significant drawback for total weekly Kt/V interpretation in PD. For instances, underweight and overweight patients should have their V calculated with their ideal body weight, according to the CSN Guidelines/Recommendations19.
Debowska and colleagues has also proposed Kt as a potential auxiliary index in PD, as Kt/V depends strongly on body size82. Currently in clinical practice, however, V is estimated from the Watson or Hume equation in adults79. All the guidelines propose a weekly total (urinary + peritoneal) Kt/V of 1.7 as a minimum target (grade A), as expressed in Table 1.
The ADEMEX10 and the Hong Kong83 randomized controlled trials found no improvement in patient survival with peritoneal Kt/V above 1.7. So, in anuric patients peritoneal Kt/V should be greater than 1.7. It is important to note that both RA and ISPD guidelines recommend increasing dialysis dose for patients experiencing uremic symptoms, even if they accomplish the minimum clearance targets (grade 1B)17,20. The discrepancy between small and larger molecules clearance leads the same guidelines to propose another recommendation, in favour of continuous regimens particularly in anuric patients (grade 1B)17,20.
The other solute removal index currently being used in PD practice is weekly creatinine clearance, adjusted to 1.73m2. The caveat of this method relates to the major impact of peritoneal membrane transport characteristics in creatinine clearance. Although the ISPD workgroup stated it was a superfluous to use creatinine clearance, the guidelines/recommendations recommended an additional target of 45 L/week/1.73m2 in APD (grade C)17.
The current clearance targets (weekly Kt/V and creatinine clearance) are attainable with easiness, particularly if the patients maintain significant residual renal function and/or are using Automated Peritoneal Dialysis. Conversely, if the targets are not achieved, patients should be monitored carefully for signs of overhydration, uremic complaints and malnutrition. Appropriate therapy changes might be considered (grade C)17. Elizabeth Oei noticed that Kt/V targets >1.7 are based on studies that included very few elderly patients (> 80 yrs-old). Escalate PD dose may be counterproductive in elderly and can increase technique failure84.
Both KT/V urea and creatinine clearance are useful but limited tool of adequacy quantifying only small solute removal and neglecting medium molecules and uremic toxins removal. However, creatinine peritoneal clearance is particularly useful to take into account in APD patients and slower transporters. In those patients, “adequate” KT/V urea might be achieved but a slower transport of creatinine or phosphorus compromises its removal. By measuring creatinine peritoneal clearance, which correlates with phosphate clearance, several optimization steps such as increasing dwell times or adjusting number of cycles, for example, can be taken.
It must be highlighted, however, that adequacy of dialysis should be interpreted clinically (clinical and laboratory results, hydration status, energy level, anemia control, electrolytes and acid-base balance, blood pressure control) rather than by targeting only solute and fluid removal. During the monthly evaluation of PD patients, nutritional status should be estimated. Serum albumin should be monitored, and when obtaining 24-hour total solute clearances, dietary protein intake estimations (DPI; such as nPNA) should be measured79. Even more, serum albumin must be viewed in the patient context (peritoneal membrane transport type, total solute clearance, volume status, comorbid diseases and any inflammatory state)79. Several investigators proposed that DPI should be in the 0.9 to 1.1 g/Kg/day range85,86. When considering DPI targets however, it should be taken into account that level of evidence is limited and targets were extrapolated from predialysis populations. In dialysis, catabolism rate and metabolic demand is variable, so patients and modality variables confound PNA.
More than PNA, also body composition (BIA) variables such as body cell mass or lean body mass and fat mass would be preferable to evaluate nutritional status. Many PD patients supposedly with malnutrition due to PNI < 1.1 g/Kg/day actually maintain age adjusted lean body mass. It might also be questionable if same targets should be applied to elderly patients. Also, it is essential to emphasize the frequency of solute clearance and the importance of renal residual function.
1. Frequency of solute clearance (renal and peritoneal) estimation
Both residual renal and peritoneal dialysis small solute clearance should be evaluated at least every 6 months, or even more often if they are dependent on residual renal function. The urea and/or creatinine clearance can be used indistinctly, but should be interpreted within their limits (grade 1C)20. The CSN guidelines proposed a measurement of total Kt/V 4-6 weeks after initiation of PD and thereafter it should be repeated whenever there is an unexplained change in the patient’s clinical status or a problem with ultrafiltration (opinion)19.
The Portuguese Good Practice Manual (PGPM) in Chronic Dialysis solutes clearance recommends weekly Kt/V evaluation every 4 months80. It should be noted that all measurements of peritoneal solute clearance should be obtained when the patient is clinically stable and at least one month after resolution of an episode of peritonitis79.
2. Residual renal function
Urine volume higher than 100 ml/day is considered significant. A mean of 24-hour urine urea clearance and urine creatinine clearance express RRF (grade D)79.
The ISPD guidelines recommend monitoring RRF every 1–2 months if practicable, but no less than every 4–6 months, for patients that rely significantly in that component of the total small solute clearance (grade C)17. The CSN guidelines indicate that RRF should be measured every 3–6 months in patients with peritoneal Kt/V <1.7 weekly and together with pKt/V when clinically indicated in all other patients (pKt/V≥1.7; grade D)19. Studies of the adequacy of PD, measured by Kt/Vurea and Ccr, have shown that in the presence of RRF outcome is driven only by the kidney component(87-90).
Guidelines on Nutrition
When nutrition is discussed, there are two opposite situations that require attention: sarcopenic-obesity and protein-energy wasting (PEW).
In 2008, the International Society of Renal Nutrition and Metabolism (ISRNM) defined PEW as a state of decreased protein and energy body stores91. PEW has been associated with major adverse clinical outcomes, increased rates of hospitalization and death92. Prevalence of PEW is variable (18-75%)93, and it is not clear if PD patients are more affected than hemodialysis patients. Some studies account seric albumin as a marker of PEW in PD patients, overestimating its prevalence in PD94. Seric albumin in dialysis patients is related to hydration status and inflammation and reflects loss of somatic protein only at late stage of PEW. Additionally, in PD patients a low albumin value may signify a greater loss of this protein without greater PEW risk.
The other aspect of nutrition is sarcopenic-obesity. Although this spectrum of nutrition is often neglected, including by the existing guidelines, some studies suggest that overweight is a more relevant problem than PEW in PD95 Obesity is not correlated with peritoneal transport or glucose absorption but is clearly associated with insulin resistance96. Some authors suggest that cumulative exposure to glucose solutions used in PD might lead to systemic hyperglycemia, insulinresistance and obesity, which can contribute to increased cardiovascular risk. Here, we will review four essential aspects of nutrition recommendations related to PEW, since sarcopenic-obesity is neglected by existing guidelines.
1. Nutritional counseling
It is advisable that all dialysis patients should receive nutritional counseling (ERBP: Evidence C; KDOQI: Opinion)(97,98). In 2000, KDOQI recommendations suggested following an individualized plan before or during the early phase of dialysis therapy, to be updated at least every three to four months98. There are no more recent guidelines regarding this matter.
2. Nutritional assessment
In terms of nutritional assessment there are two important questions that need to be addressed: when and how it is to be carried out? The most recent guidelines in this respect are from the ISPD and recommend that a nutritional assessment of PD patients be made during the period of six to eight weeks after initiation of the technique and then regularly, at least every four to six months (ungraded)78.
There is no tool that can, of itself, reliably assess nutritional status, which is why the use of a combination of various tools is recommended (KDOQI: opinion)98. In 2005, ERBP guidelines acknowledged that serum albumin alone was not a clinically useful measure of nutritional assessment in PD patients (Evidence B). These guidelines recommend the use of subjective global assessment, protein intake (assessed from the protein equivalent of total nitrogen appearance, nPNA, or by dietary recall) and an assessment of protein nutrition (Evidence C)97 to assess nutritional patient status. The diagnosis of PEW was proposed by the ISRNM based on serum chemistry, body and muscle mass and dietary intake91.
More recently, bioimpedance has been used in several studies to assess patient body composition and has shown some relationship with patient survival99. However, over the last ten years, nutrition guidelines in dialysis patients have not been reviewed and this tool has not yet been integrated into the international guidelines.
3. PEW prevention
PEW prevention includes adequate protein and energy intake. The most recent recommendations date from 2013 (ISRNM Consensus)94 and recommend a protein intake higher than 1.2g/kg/day (higher than 1.5g/kg/day during peritonitis episodes), more than 50% of which must be of high biological value, and an energetic intake of 30-35 kcal/kg/day (including kcal from dialysate), both based on ideal body weight. For the sedentary elderly, the recommended energy intake, based on physical activity level, is only 30 kcal/kg/day. ISRNM Consensus also advocates a minimum dialysis dose for maintaining adequate intake and preserving nutritional status94. However, it should be noted that increasing dialysis dose beyond the recommended did not show efficacy in improving nutritional status10.
The same document proposes additional measures for PEW prevention, including metabolic acidosis correction until the level of bicarbonate is higher than 22 (with increased dialysis dose and/or oral bicarbonate), and the reduction of systemic inflammation through early treatment of inflammatory conditions and adequate volume control94.
4. PEW treatment
Before any therapeutic attitude, detection and correction of reversible causes are mandatory (ERBP: Evidence C)97. Exclusion of any causes other than those related to dialysis technique (dialysis inadequacy and metabolic acidosis), namely, psychiatric, socioeconomic and other medical causes (gastroparesis, malabsorption, early satiety, inflammation) are warranted. Treatment of PEW is accomplished with nutritional support. Ideally, oral diet may be reinforced with energy and protein supplements98.
This intake should be carried out two to three times a day, after meals, over a period from three to twelve months, to provide an additional energy intake of 7-10 Kcal/kg/day and protein of 0.3-0.4g/kg/day. For patients who are unable to tolerate nutritional supplementation by mouth, tube feeding should be considered98,100.
Intraperitoneal amino acid solutions have been described as an alternative to treatment of malnutrition in PD patients. Recommendations are contradictory19,98,100, although the most recent consensus is in favor of their use100. If intraperitoneal amino acid solutions are used, more than one daily exchange is not recommended (ISPD: grade A) and monitoring of acidosis and uremia (ISPD: grade B) is required19. Although this measure appears to have little impact in PEW treatment, it may be an important weapon, along with use of icodextrin solutions and preservation of residual renal function, as a glucose-sparing strategy in obese patients. Finally, the use of anabolic steroids (nandrolone decanoate 100mg intramuscular injection weekly) for up to six months (ISPD: grade B) or the appetite stimulant megestrol acetate (ISPD: grade D) should be considered to improve nutritional condition19,101.
Guidelines on Cardiovascular and metabolic risk
Adult peritoneal dialysis patients present high cardiovascular morbidity and mortality, since coronary artery disease (CAD), left ventricular hypertrophy (LVH), heart failure (HF), atrial fibrillation (AF), cerebrovascular disease (CVD) and peripheral artery disease (PAD) are highly prevalent in this population. Thus, it is essential to identify interventions that can lead to reduce cardiovascular mortality and enhanced survival amongst these patients. Currently, the available evidence is not enough to determine the optimal approach in some areas, highlighting the need for further studies.
1. Coronary artery disease
The diagnosis of acute coronary syndrome (ACS) requires the determination of biomarkers of cardiac injury, such as troponin I and T. Its reference range is difficult to define in the general population and even more so in dialysis patients, since they are frequently elevated even in the absence of an acute coronary event102,103. The underlying pathophysiological mechanism is not entirely understood; several hypotheses including the influence of different laboratory assays104,105 and residual renal function106,107 have been proposed, with studies available showing conflicting results. Nonetheless, it has been noted that elevated cardiac biomarkers correlate with myocardial leak102 – even after adjusting for residual renal function and the type of depurative technic used – which implies underlying cardiac disease104,108. Troponin elevation in these patients may indicate variable cardiac disease and not necessarily acute ischemic events103,105,109.
A single elevated value has little or no diagnostic utility; however, it may be of potential interest for cardiovascular risk stratification105,109,110. Studies in PD suggest an association between elevated troponin T and LVH, global and cardiovascular mortality105. Elevated troponin T predicts long-term mortality, cardiovascular events and cardiovascular mortality103,104,110-112. There is insufficient data to recommend its routine use; nonetheless, in case of incidental detection of raised troponin, these patients may benefit from additional investigation to detect silent cardiac disease, such as LVH, CAD orsystolic dysfunction. Conversely, high troponin detected in serial measurements in the appropriated clinical setting establishes the diagnosis of ACS – the difficulty in this subset of patients is to define the range113.
Therefore, in the presence of acute symptoms along with electrocardiographic changes or any other clinical alteration suggestive of acute myocardial ischemia, a serial measurement of cardiac biomarkers should be undertaken to evaluate a possible ACS. A rise in troponin level > 20%, with at least one value above 99th percentile, within 4-9h is diagnostic114,115.
Cardiovascular disease in end-stage renal disease (ESRD) is underdiagnosed and undertreated, since its signs and symptoms are often subtle116,117. Routine screening has been considered in this population; however there is not enough evidence to support it. A careful history and physical examination are sufficient to identify patients with high pretest probability of having significant cardiac disease, without potential adverse effects or additional costs114,117. In renal transplant candidates, further investigation with non-invasive stress tests should be carried out if there are three or more of the following risk factors: diabetes, previous history of cardiovascular disease, more than one year on dialysis, LVH, age above 60 years, smoking, hypertension and dyslipidemia114,117. The above criteria were based upon the Lisbon Conference Report 2007 recommendations and represent an alternative to the ones defined by the American Heart Association and the American College of Cardiology for the general population, with higher sensitivity and specificity in chronic kidney disease (CKD)118.
After the diagnosis, patients with CKD are less likely to be prescribed anti-platelet agents – only about 27-49%116,119,120. Studies in PD are lacking; however observational studies in hemodialysis (HD) have shown that only about half the patients (51%) with indication were on acetylsalicylic acid for primary and secondary prevention of CAD, and amongst the ones not taking it, 53% had clear indication without identified contraindication121. A meta-analysis that included 17 studies with ESRD patients concluded that anti-platelet agents reduce the risk of acute myocardial infarction (AMI)122. Thus, in the absence of an increased bleeding risk, PD patients should be treated the same way as the general population114.
Current guidelines suggest that dyslipidemia management in PD patients should follow the KDIGO recommendations78. An initial evaluation of lipid profile should be performed; however monitoring its levels is not required. If a patient is already on pharmacological therapy, then it should be maintained; otherwise, it should not be initiated123.
2. Left ventricular hypertrophy and dysfunction |Heart failure
LVH, systolic dysfunction and heart failure are highly prevalent in PD patients – reported in over 90, 16 and 39%, respectively124. It translates into high morbidity and mortality, since the presence of systolic dysfunction is associated with a higher risk of heart failure and sudden death, and they all predict mortality(125, 126). Hence, an echocardiogram should be performed when starting PD and whenever a clinical alteration is detected, to evaluate for LVH, dilatation, systolic and diastolic functions, as well as cardiac valvular abnormalities. In patients with significant systolic dysfunction, further investigation to exclude possible CAD is recommended114.
Data regarding the use of several drugs frequently prescribed to the general population suggest they may also be beneficial in PD patients. The use of angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB) and beta blockers (BB) should be considered in PD patients with LVH, dilated cardiomyopathy or systolic heart failure; when already receiving treatment with ACEI or ARB, consider adding a mineralocorticoid receptor antagonist (MRA)114. Observational studies showed that ACEI/ARB utilization is associated with a reduction in cardiovascular events, and cardiovascular and all-cause mortality, even after adjusting for other risk factors and patient characteristics127,128. Concerning the treatment with MRA, a randomized controlled trial (RCT) in PD showed that, when added to a ACEI or ARB, spironolactone significantly reduces the rate of progression in left ventricular mass index and improves ejection fraction for 24 months129. Studies evaluating the influence of BB in LVH, systolic dysfunction and HF related outcomes in PD patients are lacking. However, in HD patients, its use improved left ventricular remodeling and functional class in patients with dilated cardiomyopathy sustained for a period of 24 months and showed significant benefit in survival, cardiovascular mortality, fatal AMI, stroke, HF related and all-cause hospitalizations130.
The optimal hemoglobin target level when treating anemia and its prognosis in PD patients with HF remains elusive. RCTs in HD have failed to demonstrate greater regression of LVH and dilatation with higher values(131, 132). On the other hand, an observational study including PD patients found that a target below 11-12 g/dL was associated with higher cardiovascular and global mortality133. There is insufficient data to recommend a dissimilar approach and hemoglobin goals in patients with or without HF114.
3. Cerebrovascular disease
Screening for CVD in PD patients is not recommended; however, a carotid duplex ultrasonography should be performed in those presenting with a transient ischemic attack (TIA) or stroke, since it allows the identification of modifiable abnormalities with prognostic significance114,134.
The use of anti-platelet agents for stroke prevention in high-risk patients is well established in the general population. In patients with ESRD, the evidence of benefit is scanty, and its use significantly increases the risk of major bleeding122,135. Therefore, these agents should not be prescribed for primary prevention114. Most studies available regarding treatment with warfarin excluded dialysis patients. The remaining few including individuals with CKD have failed to demonstrate a reduction in the combined risk of ischemic and hemorrhagic stroke136, with a significant increase in bleeding events137. Recently, a retrospective observational study in PD evaluated warfarin use and its impact in ischemic stroke prevention in the presence of AF. Anticoagulation was associated with less cerebrovascular events whencompared with aspirin or no treatment, without an increased risk of intracranial hemorrhage138. Giving the inconsistency of available results, its prescription should be individualized considering the increased risk of bleeding and uncertain effects on cerebrovascular outcomes114. In the absence of clinical trials including this subset of patients, the use of new/direct oral anticoagulants is not recommended for thromboembolic stroke prevention in PD with AF114.
Potential benefits and complications of thrombolytic therapy have not been evaluated specifically in PD patients139. Observational data in ESRD suggest similar risk of bleeding as in the general population; however, it is associated with adjusted higher in-hospital mortality, length of stay and in-hospital complications140. Therefore, caution is necessary since it is not clear if benefits outweigh risks114.
4. Peripheral artery disease
About 27-31% of all PD patients have PAD and, in those above 70 years old, its prevalence can be as high as 45%141,142. PAD is independently associated with cardiovascular and overall mortality143. Studies comparing routine screening vs. evaluation in the presence of clinical signs and symptoms are absent in dialysis patients; therefore, the guidelines recommend similar assessment and treatment as for the general population114.
Generally, the diagnosis is made by an ankle-brachial pressure index (ABI) < 0.9. Although highly sensitive and specific for the general population (95 and 100% respectively), its performance has not been studied in CKD patients114. Considering the high prevalence of vascular calcifications in this subset of patients, the toe-brachial index (TBI) has presented as an alternative, since smaller vessels are less likely to be affected. An ABI > 1.3 or a systolic blood pressure measured in the leg more than 20% the one evaluated in the arm suggest the presence of non-compressible vessels; and therefore, the ABI may be unreliable. A TBI (which is diagnostic if < 0.6) may be indicated in those cases144,145. This tool has been validated for the diagnosis of PAD in PD; still studies concerning its sensitivity and specificity, clinical performance (vs. doppler or angiography), and predictive value for vascular events and complications remain to be carried out 146.
Even though no solid evidence exists in ESRD, supervised exercise therapy is recommended in the absence of critical disease114. Antiplatelet therapy should be considered in PD patients according to the same indications accepted for the general population, always keeping in mind the possible augmented risk of bleeding114,147. An observational study in PD suggests reduced amputations with multidisciplinary foot care148 – regular foot evaluation, podiatrist treatment and awareness of home foot care114.
5. Cardiac arrhythmias
AF is highly prevalent in dialysis patients (between 12.5-27% in HD)114,149,150, with a higher frequency than in the general population149. It is also associated with higher mortality150,151. Thus, a 12-lead electrocardiogram should be performed in all patients starting PD and then annually, to screen for cardiac arrhythmias including AF114. As mentioned previously, hypocoagulation in this subset of patients should be individualized given the inconsistent results of available studies113,136-138.
6. Sudden cardiac death
Sudden cardiac death (SCD) accounts for about one-forth of all deaths in PD152. It has been associated with raised troponins and N-terminal pro-brain natriuretic peptide126. The presence of heart failure and systolic dysfunction are predictive factors of its occurrence126, and a previous episode of tachyarrhythmic cardiac arrest is associated with increased risk of SCD in HD patients153. Therefore, patients with low ejection fraction, elevated troponins and N-terminal pro-brain natriuretic peptide, and that have survived a previous tachyarrhythmic cardiac arrest should be considered as being at high risk of SCD114.
There are no studies regarding therapeutic interventions in PD, or RCT with antiarrhythmics for the prevention of SCD, or evaluating the efficacy of implantable cardioverter (ICD) in primary prevention in patients on dialysis. Observational studies in HD suggest a reduced number of events when using BB in patients with CAD154 and a benefit of ICD for secondary prevention of SCD.155 Hence, current expert opinion is that BB should be consider for primary prevention of SCD in high risk PD patients, and ICD for secondary prevention in patients with a previous episode of cardiac arrest as a consequence of a malignant ventricular arrhythmia (except those occurring in the first 48h after AMI)114.
CONCLUSIONS
Solutes and fluid removal are key points in PD patients treatment. Both, when optimized, are of utmost importance for maintaining patients asymptomatic and to extend in time PD treatment. RRF is as important as dialysis dose. In fact, RRF is one of the most important factors that predict survival in PD patients, and its preservation is fundamental in the follow-up of these patients. Cardiovascular events are frequent and primary prevention must be considered in PD patients in line with the same indications accepted for the general population.
References
1. Yu X, Mehrotra R, Yang X. Components of A successful peritoneal dialysis program. Semin Nephrol. 2017;37(1):10-6. [ Links ]
2. Konings CJ, Kooman JP, Schonck M, Dammers R, Cheriex E, Palmans Meulemans AP, et al. Fluid status, blood pressure, and cardiovascular abnormalities in patients on peritoneal dialysis. Perit Dial Int. 2002;22(4):477-87. [ Links ]
3. Enia G, Mallamaci F, Benedetto FA, Panuccio V, Parlongo S, Cutrupi S, et al. Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant. 2001;16(7):1459-64. [ Links ]
4. Demirci MS, Demirci C, Ozdogan O, Kircelli F, Akcicek F, Basci A, et al. Relations between malnutrition-inflammation-atherosclerosis and volume status. The usefulness of bioimpedance analysis in peritoneal dialysis patients. Nephrol Dial Transplant. 2011;26(5):1708-16. [ Links ]
5. Paniagua R, Ventura MD, Avila-Diaz M, Hinojosa-Heredia H, Mendez-Duran A, Cueto-Manzano A, et al. NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant. 2010;25(2):551-7. [ Links ]
6. Van Biesen W, Williams JD, Covic AC, Fan S, Claes K, Lichodziejewska-Niemierko M, et al. Fluid status in peritoneal dialysis patients: the European Body Composition Monitoring (EuroBCM) study cohort. PLoS One. 2011;6(2):e17148. [ Links ]
7. Devolder I, Verleysen A, Vijt D, Vanholder R, Van Biesen W. Body composition, hydration, and related parameters in hemodialysis versus peritoneal dialysis patients. Perit Dial Int. 2010;30(2):208-14. [ Links ]
8. Juan-Garcia I, Puchades MJ, Sanjuan R, Torregrosa I, Solis MA, Gonzalez M, et al. Echocardiographic impact of hydration status in dialysis patients. Nefrologia. 2012;32(1):94-102. [ Links ]
9. Kwan BC, Szeto CC, Chow KM, Law MC, Cheng MS, Leung CB, et al. Bioimpedance spectroscopy for the detection of fluid overload in Chinese peritoneal dialysis patients. Perit Dial Int. 2014;34(4):409-16. [ Links ]
10. Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13(5):1307-20. [ Links ]
11. Bargman JM, Thorpe KE, Churchill DN, Group CPDS. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12(10):2158-62. [ Links ]
12. Ates K, Nergizoglu G, Keven K, Sen A, Kutlay S, Erturk S, et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int. 2001;60(2):767-76. [ Links ]
13. Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, et al. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD Outcome Study. J Am Soc Nephrol. 2003;14(11):2948-57. [ Links ]
14. Jansen MA, Termorshuizen F, Korevaar JC, Dekker FW, Boeschoten E, Krediet RT, et al. Predictors of survival in anuric peritoneal dialysis patients. Kidney Int. 2005;68(3):1199-205. [ Links ]
15. Lin X, Lin A, Ni Z, Yao Q, Zhang W, Yan Y, et al. Daily peritoneal ultrafiltration predicts patient and technique survival in anuric peritoneal dialysis patients. Nephrol Dial Transplant. 2010;25(7):2322-7. [ Links ]
16. Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, et al. Evaluation and management of ultrafiltration problems in peritoneal dialysis. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000;20(4):S5-21. [ Links ]
17. Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, et al. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int. 2006;26(5):520-2. [ Links ]
18. Dombros N, Dratwa M, Feriani M, Gokal R, Heimburger O, Krediet R, et al. European best practice guidelines for peritoneal dialysis. 7 Adequacy of peritoneal dialysis. Nephrol Dial Transplant. 2005;20(9):ix24-ix7. [ Links ]
19. Blake PG, Bargman JM, Brimble KS, Davison SN, Hirsch D, McCormick BB, et al. Clinical Practice Guidelines and Recommendations on Peritoneal Dialysis Adequacy 2011. Perit Dial Int. 2011;31(2):218-39. [ Links ]
20. Woodrow G, Fan SL, Reid C, Denning J, Pyrah AN. Renal Association Clinical Practice Guideline on peritoneal dialysis in adults and children. BMC Nephrol. 2017;18(1):333. [ Links ]
21. Davies SJ. Overfill or ultrafiltration? We need to be clear. Perit Dial Int. 2006;26(4):449-51. [ Links ]
22. Ronco C, Verger C, Crepaldi C, Pham J, De Los Rios T, Gauly A, et al. Baseline hydration status in incident peritoneal dialysis patients: the initiative of patient outcomes in dialysis (IPOD-PD study) dagger. Nephrol Dial Transplant. 2015;30(5):849-58. [ Links ]
23. Garg R, Singh A, Khaja A, Martin A, Aggarwal K. How does volume status affect BNP and troponin levels as markers of cardiovascular status in peritoneal dialysis? Congest Heart Fail. 2009;15(5):240-4. [ Links ]
24. Panuccio V, Enia G, Tripepi R, Torino C, Garozzo M, Battaglia GG, et al. Chest ultrasound and hidden lung congestion in peritoneal dialysis patients. Nephrol Dial Transplant. 2012;27(9):3601-5. [ Links ]
25. OLone EL, Visser A, Finney H, Fan SL. Clinical significance of multi-frequency bioimpedance spectroscopy in peritoneal dialysis patients: independent predictor of patient survival. Nephrol Dial Transplant. 2014;29(7):1430-7. [ Links ]
26. Luo YJ, Lu XH, Woods F, Wang T. Volume control in peritoneal dialysis patients guided by bioimpedance spectroscopy assessment. Blood Purif. 2011;31(4):296-302. [ Links ]
27. Clinical practice recommendations for peritoneal dialysis adequacy. Am J Kidney Dis. 2006;48(1):S130-58. [ Links ]
28. Johnson D, Brown F, Lammi H, Walker R, Caring for Australians with Renal I. The CARI guidelines. Dialysis adequacy (PD) guidelines. Nephrology (Carlton). 2005;10(4):S81-107. [ Links ]
29. van Biesen W, Heimburger O, Krediet R, Rippe B, La Milia V, Covic A, et al. Evaluation of peritoneal membrane characteristics: clinical advice for prescription management by the ERBP working group. Nephrol Dial Transplant. 2010;25(7):2052-62. [ Links ]
30. Manual de Boas Práticas da Diálise Crónica, (2017). [ Links ]
31. La Milia V, Pozzoni P, Virga G, Crepaldi M, Del Vecchio L, Andrulli S, et al. Peritoneal transport assessment by peritoneal equilibration test with 3.86% glucose: a long-term prospective evaluation. Kidney Int. 2006;69(5):927-33. [ Links ]
32. Sampimon DE, Coester AM, Struijk DG, Krediet RT. Time course of peritoneal transport parameters in peritoneal dialysis patients who develop peritoneal sclerosis. Adv Perit Dial. 2007;23:107-11. [ Links ]
33. La Milia V, Virga G, Amici G, Bertoli S, Cancarini G. Functional assessment of the peritoneal membrane. J Nephrol. 2013;26(21):120-39. [ Links ]
34. Fernstrom A, Hylander B, Moritz A, Jacobsson H, Rossner S. Increase of intra-abdominal fat in patients treated with continuous ambulatory peritoneal dialysis. Perit Dial Int. 1998;18(2):166-71. [ Links ]
35. Van V, Schoonjans RS, Struijk DG, Verbanck JJ, Vanholder RC, Van B, et al. Influence of dialysate on gastric emptying time in peritoneal dialysis patients. Perit Dial Int. 2002;22(1):32-8. [ Links ]
36. Smit W, Schouten N, van den Berg N, Langedijk MJ, Struijk DG, Krediet RT, et al. Analysis of the prevalence and causes of ultrafiltration failure during long-term peritoneal dialysis: a crosssectional study. Perit Dial Int. 2004;24(6):562-70. [ Links ]
37. Davies SJ, Brown EA, Frandsen NE, Rodrigues AS, Rodriguez-Carmona A, Vychytil A, et al. Longitudinal membrane function in functionally anuric patients treated with APD: data from EAPOS on the effects of glucose and icodextrin prescription. Kidney Int. 2005;67(4):1609-15. [ Links ]
38. Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int. 1994;46(2):496-503. [ Links ]
39. Finkelstein F, Healy H, Abu-Alfa A, Ahmad S, Brown F, Gehr T, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol. 2005;16(2):546-54. [ Links ]
40. Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol. 2003;14(9):2338-44. [ Links ]
41. Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int. 2003;63(4):1556-63. [ Links ]
42. Cho Y, Johnson DW, Craig JC, Strippoli GF, Badve SV, Wiggins KJ. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev. 2014(3):CD007554. [ Links ]
43. Sav T, Oymak O, Inanc MT, Dogan A, Tokgoz B, Utas C. Effects of twice-daily icodextrin administration on blood pressure and left ventricular mass in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2009;29(4):443-9. [ Links ]
44. Dousdampanis P, Trigka K, Chu M, Khan S, Venturoli D, Oreopoulos DG, et al. Two icodextrin exchanges per day in peritoneal dialysis patients with ultrafiltration failure: one centers experience and review of the literature. Int Urol Nephrol. 2011;43(1):203-9. [ Links ]
45. Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17(6):1085-92. [ Links ]
46. Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1998;9(7):1285-92. [ Links ]
47. Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol. 2006;17(1):271-8. [ Links ]
48. Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol. 2006;17(9):2591-8. [ Links ]
49. Johnson DW, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Superior survival of high transporters treated with automated versus continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2010;25(6):1973-9. [ Links ]
50. Davies SJ. Mitigating peritoneal membrane characteristics in modern peritoneal dialysis therapy. Kidney Int Suppl. 2006(103):S76-83. [ Links ]
51. Rodriguez-Carmona A, Perez-Fontan M, Garca-Naveiro R, Villaverde P, Peteiro J. Compared time profiles of ultrafiltration, sodium removal, and renal function in incident CAPD and automated peritoneal dialysis patients. Am J Kidney Dis. 2004;44(1):132-45. [ Links ]
52. Rodriguez-Carmona A, Fontan MP. Sodium removal in patients undergoing CAPD and automated peritoneal dialysis. Perit Dial Int. 2002;22(6):705-13. [ Links ]
53. Konings CJ, Kooman JP, Schonck M, Struijk DG, Gladziwa U, Hoorntje SJ, et al. Fluid status in CAPD patients is related to peritoneal transport and residual renal function: evidence from a longitudinal study. Nephrol Dial Transplant. 2003;18(4):797-803. [ Links ]
54. Szeto CC, Kwan BC, Chow KM, Chung S, Yu V, Cheng PM, et al. Predictors of residual renal function decline in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 2015;35(2):180-8. [ Links ]
55. Li PK, Chow KM, Wong TY, Leung CB, Szeto CC. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med. 2003;139(2):105-12. [ Links ]
56. Suzuki H, Kanno Y, Sugahara S, Okada H, Nakamoto H. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis. 2004;43(6):1056-64. [ Links ]
57. Zhang L, Zeng X, Fu P, Wu HM. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for preserving residual kidney function in peritoneal dialysis patients. Cochrane Database Syst Rev. 2014(6):CD009120. [ Links ]
58. Medcalf JF, Harris KP, Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001;59(3):1128-33. [ Links ]
59. McCafferty K, Fan S, Davenport A. Extracellular volume expansion, measured by multifrequency bioimpedance, does not help preserve residual renal function in peritoneal dialysis patients. Kidney Int. 2014;85(1):151-7. [ Links ]
60. Van Biesen W, Jorres A. Fluid overload and residual renal function in peritoneal dialysis: the proof of the pudding is in the eating. Kidney Int. 2014;85(1):15-7. [ Links ]
61. Liao CT, Shiao CC, Huang JW, Hung KY, Chuang HF, Chen YM, et al. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int. 2008;28(3):S191-5. [ Links ]
62. Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62(3):1046-53. [ Links ]
63. Shemin D, Maaz D, St Pierre D, Kahn SI, Chazan JA. Effect of aminoglycoside use on residual renal function in peritoneal dialysis patients. Am J Kidney Dis. 1999;34(1):14-20. [ Links ]
64. Baker RJ, Senior H, Clemenger M, Brown EA. Empirical aminoglycosides for peritonitis do not affect residual renal function. Am J Kidney Dis. 2003;41(3):670-5. [ Links ]
65. Moranne O, Willoteaux S, Pagniez D, Dequiedt P, Boulanger E. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21(4):1040-5. [ Links ]
66. Dittrich E, Puttinger H, Schillinger M, Lang I, Stefenelli T, Horl WH, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients--a prospective study. Nephrol Dial Transplant. 2006;21(5):1334-9. [ Links ]
67. Breborowicz A, Pawlaczyk K, Polubinska A, Gorna K, Wieslander A, Carlsson O, et al. Effect of peritoneal dialysis on renal morphology and function. Nephrol Dial Transplant. 2006;21(12):3539-44. [ Links ]
68. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int. 2004;66(1):408-18. [ Links ]
69. Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int. 2008;73(2):200-6. [ Links ]
70. Kim SG, Kim S, Hwang YH, Kim K, Oh JE, Chung W, et al. Could solutions low in glucose degradation products preserve residual renal function in incident peritoneal dialysis patients? A 1-year multicenter prospective randomized controlled trial (Balnet Study). Perit Dial Int. 2008;28(3):S117-22. [ Links ]
71. Haag-Weber M, Kramer R, Haake R, Islam MS, Prischl F, Haug U, et al. Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant. 2010;25(7):2288-96. [ Links ]
72. Sikaneta T, Wu G, Abdolell M, Ng A, Mahdavi S, Svendrovski A, et al. The Trio Trial - A Randomized Controlled Clinical Trial Evaluating the Effect of a Biocompatible Peritoneal Dialysis Solution on Residual Renal Function. Perit Dial Int. 2016;36(5):526-32. [ Links ]
73. Yohanna S, Alkatheeri AM, Brimble SK, McCormick B, Iansavitchous A, Blake PG, et al. Effect of Neutral-pH, Low-Glucose Degradation Product Peritoneal Dialysis Solutions on Residual Renal Function, Urine Volume, and Ultrafiltration: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol. 2015;10(8):1380-8. [ Links ]
74. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol. 2012;23(6):1097-107. [ Links ]
75. Htay H, Cho Y, Pascoe EM, Darssan D, Hawley C, Johnson DW, et al. Predictors of Residual Renal Function Decline in Peritoneal Dialysis Patients: The balANZ Trial. Perit Dial Int. 2017;37(3):283-9. [ Links ]
76. Seo EY, An SH, Cho JH, Suh HS, Park SH, Gwak H, et al. Effect of biocompatible peritoneal dialysis solution on residual renal function: a systematic review of randomized controlled trials. Perit Dial Int. 2014;34(7):724-31. [ Links ]
77. Wang J, Zhu N, Yuan W. Effect of neutral pH and low-glucose degradation product-containing peritoneal dialysis solution on residual renal function in peritoneal dialysis patients: a metaanalysis. Nephron. 2015;129(3):155-63. [ Links ]
78. Wang AY, Brimble KS, Brunier G, Holt SG, Jha V, Johnson DW, et al. ISPD Cardiovascular and Metabolic Guidelines in Adult Peritoneal Dialysis Patients Part I - Assessment and Management of Various Cardiovascular Risk Factors. Perit Dial Int. 2015;35(4):379-87.
79. Peritoneal Dialysis Adequacy Work G. Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. 2006;48(1):S98-129. [ Links ]
80. Manual de Boas Práticas de Diálise Crónica da Ordem dos Médicos, (2017). [ Links ]
81. Wang L, Wang T. Adequacy of peritoneal dialysis: Kt/V revisited. Eur Rev Med Pharmacol Sci. 2015;19(7):1272-5. [ Links ]
82. Debowska M, Paniagua R, Ventura MD, Avila-Diaz M, Prado-Uribe C, Mora C, et al. Dialysis adequacy indices and body composition in male and female patients on peritoneal dialysis. Perit Dial Int. 2014;34(4):417-25. [ Links ]
83. Lo WK, Ho YW, Li CS, Wong KS, Chan TM, Yu AW, et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int. 2003;64(2):649-56. [ Links ]
84. Oei E, Fan S. Peritoneal Dialysis Adequacy in Elderly Patients. Perit Dial Int. 2015;35(6):635-9. [ Links ]
85. Lim VS, Flanigan MJ. Protein intake in patients with renal failure: comments on the current NKFDOQI guidelines for nutrition in chronic renal failure. Semin Dial. 2001;14(3):150-2. [ Links ]
86. Uribarri J, Levin NW, Delmez J, Depner TA, Ornt D, Owen W, et al. Association of acidosis and nutritional parameters in hemodialysis patients. Am J Kidney Dis. 1999;34(3):493-9. [ Links ]
87. Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999;33(3):523-34. [ Links ]
88. Jager KJ, Merkus MP, Dekker FW, Boeschoten EW, Tijssen JG, Stevens P, et al. Mortality and technique failure in patients starting chronic peritoneal dialysis: results of The Netherlands Cooperative Study on the Adequacy of Dialysis. NECOSAD Study Group. Kidney Int. 1999;55(4):1476-85. [ Links ]
89. Szeto CC, Wong TY, Leung CB, Wang AY, Law MC, Lui SF, et al. Importance of dialysis adequacy in mortality and morbidity of chinese CAPD patients. Kidney Int. 2000;58(1):400-7. [ Links ]
90. Rocco M, Soucie JM, Pastan S, McClellan WM. Peritoneal dialysis adequacy and risk of death. Kidney Int. 2000;58(1):446-57. [ Links ]
91. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391-8. [ Links ]
92. Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80(2):299-307. [ Links ]
93. Mehrotra R, Kopple JD. Nutritional management of maintenance dialysis patients: why arent we doing better? Annu Rev Nutr. 2001;21:343-79. [ Links ]
94. Park YK, Kim JH, Kim KJ, Seo AR, Kang EH, Kim SB, et al. A cross-sectional study comparing the nutritional status of peritoneal dialysis and hemodialysis patients in Korea. J Ren Nutr. 1999;9(3):149-56. [ Links ]
95. Bernardo AP, Fonseca I, Rodrigues A, Carvalho MJ, Cabrita A. Overweight rather than malnutrition is widely prevalent in peritoneal dialysis patients. Adv Perit Dial. 2009;25:119-24. [ Links ]
96. Bernardo AP, Oliveira JC, Santos O, Carvalho MJ, Cabrita A, Rodrigues A. Insulin Resistance in Nondiabetic Peritoneal Dialysis Patients: Associations with Body Composition, Peritoneal Transport, and Peritoneal Glucose Absorption. Clin J Am Soc Nephrol. 2015;10(12):2205-12. [ Links ]
97. Dombros N, Dratwa M, Feriani M, Gokal R, Heimburger O, Krediet R, et al. European best practice guidelines for peritoneal dialysis. 8 Nutrition in peritoneal dialysis. Nephrol Dial Transplant. 2005;20(9):ix28-ix33. [ Links ]
98. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35( 2):S1-140. [ Links ]
99. Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, et al. Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol. 2015;10(7):1192-200. [ Links ]
100. Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84(6):1096-107. [ Links ]
101. Ordem dos Médicos - Código Deontológico. Regulamento nº 14/2016. Diário da República nº139; 21 de Julho de 2016. p. 22582. [ Links ]
102. Abaci A, Ekici E, Oguzhan A, Tokgoz B, Utas C. Cardiac troponins T and I in patients with end-stage renal disease: the relation with left ventricular mass and their prognostic value. Clin Cardiol. 2004;27(12):704-9. [ Links ]
103. Lowbeer C, Gutierrez A, Gustafsson SA, Norrman R, Hulting J, Seeberger A. Elevated cardiac troponin T in peritoneal dialysis patients is associated with CRP and predicts all-cause mortality and cardiac death. Nephrol Dial Transplant. 2002;17(12):2178-83. [ Links ]
104. Ooi DS, Zimmerman D, Graham J, Wells GA. Cardiac troponin T predicts long-term outcomes in hemodialysis patients. Clin Chem. 2001;47(3):412-7. [ Links ]
105. Duman D, Tokay S, Toprak A, Duman D, Oktay A, Ozener IC, et al. Elevated cardiac troponin T is associated with increased left ventricular mass index and predicts mortality in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20(5):962-7. [ Links ]
106. Diris JH, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen-Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109(1):23-5. [ Links ]
107. Ziebig R, Lun A, Hocher B, Priem F, Altermann C, Asmus G, et al. Renal elimination of troponin T and troponin I. Clin Chem. 2003;49(7):1191-3. [ Links ]
108. Newby LK, Jesse RL, Babb JD, Christenson RH, De Fer TM, Diamond GA, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012;60(23):2427-63. [ Links ]
109. Sommerer C, Giannitsis E, Schwenger V, Zeier M. Cardiac biomarkers in haemodialysis patients: the prognostic value of amino-terminal pro-B-type natriuretic peptide and cardiac troponin T. Nephron Clin Pract. 2007;107(3):c77-81. [ Links ]
110. Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005;112(20):3088-96. [ Links ]
111. Hassan HC, Howlin K, Jefferys A, Spicer ST, Aravindan AN, Suryanarayanan G, et al. High-sensitivity troponin as a predictor of cardiac events and mortality in the stable dialysis population. Clin Chem. 2014;60(2):389-98. [ Links ]
112. Wang AY, Lam CW, Wang M, Chan IH, Goggins WB, Yu CM, et al. Prognostic value of cardiac troponin T is independent of inflammation, residual renal function, and cardiac hypertrophy and dysfunction in peritoneal dialysis patients. Clin Chem. 2007;53(5):882-9. [ Links ]
113. Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106(23):2941-5. [ Links ]
114. Wang AY, Brimble KS, Brunier G, Holt SG, Jha V, Johnson DW, et al. ISPD Cardiovascular and Metabolic Guidelines in Adult Peritoneal Dialysis Patients Part II - Management of Various Cardiovascular Complications. Perit Dial Int. 2015;35(4):388-96. [ Links ]
115. Group NW, Wu AH, Jaffe AS, Apple FS, Jesse RL, Francis GL, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53(12):2086-96. [ Links ]
116. Gowdak LH, Arantes RL, de Paula FJ, Krieger EM, De Lima JJ. Underuse of American College of Cardiology/American Heart Association Guidelines in hemodialysis patients. Ren Fail. 2007;29(5):559-65. [ Links ]
117. Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, Kasiske BL, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60(5):434-80. [ Links ]
118. Abbud-Filho M, Adams PL, Alberu J, Cardella C, Chapman J, Cochat P, et al. A report of the Lisbon Conference on the care of the kidney transplant recipient. Transplantation. 2007;83(8):S1-22. [ Links ]
119. Tonelli M, Bohm C, Pandeya S, Gill J, Levin A, Kiberd BA. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am J Kidney Dis. 2001;37(3):484-9. [ Links ]
120. Chow FY, Polkinghorne KR, Chadban SJ, Atkins RC, Kerr PG. Cardiovascular risk in dialysis patients: a comparison of risk factors and cardioprotective therapy between 1996 and 2001. Nephrology (Carlton). 2003;8(4):177-83. [ Links ]
121. Dempster DW, Rosenstock JL, Schwimmer JA, Panagopoulos G, DeVita MV, Michelis MF. Underutilization of aspirin in hemodialysis patients for primary and secondary prevention of cardiovascular disease. Clin Nephrol. 2005;64(5):371-7. [ Links ]
122. Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. 2013(2):CD008834. [ Links ]
123. Wanner C, Tonelli M, Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85(6):1303-9. [ Links ]
124. Wang AY, Wang M, Lam CW, Chan IH, Lui SF, Sanderson JE. Heart failure in long-term peritoneal dialysis patients: a 4-year prospective analysis. Clin J Am Soc Nephrol. 2011;6(4):805-12. [ Links ]
125. Wang AY, Wang M, Lam CW, Chan IH, Zhang Y, Sanderson JE. Left ventricular filling pressure by Doppler echocardiography in patients with end-stage renal disease. Hypertension. 2008;52(1):107-14. [ Links ]
126. Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE. Sudden cardiac death in end-stage renal disease patients: a 5-year prospective analysis. Hypertension. 2010;56(2):210-6. [ Links ]
127. Fang W, Oreopoulos DG, Bargman JM. Use of ACE inhibitors or angiotensin receptor blockers and survival in patients on peritoneal dialysis. Nephrol Dial Transplant. 2008;23(11):3704-10. [ Links ]
128. Shen JI, Saxena AB, Montez-Rath ME, Chang TI, Winkelmayer WC. Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use and cardiovascular outcomes in patients initiating peritoneal dialysis. Nephrol Dial Transplant. 2017;32(5):862-9. [ Links ]
129. Ito Y, Mizuno M, Suzuki Y, Tamai H, Hiramatsu T, Ohashi H, et al. Long-term effects of spironolactone in peritoneal dialysis patients. J Am Soc Nephrol. 2014;25(5):1094-102. [ Links ]
130. Cice G, Ferrara L, Di Benedetto A, Russo PE, Marinelli G, Pavese F, et al. Dilated cardiomyopathy in dialysis patients--beneficial effects of carvedilol: a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2001;37(2):407-11. [ Links ]
131. Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16(7):2180-9. [ Links ]
132. Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2000;58(3):1325-35. [ Links ]
133. Molnar MZ, Mehrotra R, Duong U, Kovesdy CP, Kalantar-Zadeh K. Association of hemoglobin and survival in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2011;6(8):1973-81. [ Links ]
134. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(1):227-76. [ Links ]
135. Caldeira D, Vaz-Carneiro A, Costa J. Cochrane Corner: evaluation of the Cochrane systematic review “antiplatelet agents for chronic kidney disease”. Rev Port Cardiol. 2013;32(11):917-8.
136. Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27(10):3816-22. [ Links ]
137. Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50(3):433-40. [ Links ]
138. Chan PH, Huang D, Yip PS, Hai J, Tse HF, Chan TM, et al. Ischaemic stroke in patients with atrial fibrillation with chronic kidney disease undergoing peritoneal dialysis. Europace. 2016;18(5):665-71. [ Links ]
139. Power A, Fogarty D, Wheeler DC. Acute stroke thrombolysis in end-stage renal disease: a national survey of nephrologist opinion. Nephron Clin Pract. 2013;124(3-4):167-72. [ Links ]
140. Tariq N, Adil MM, Saeed F, Chaudhry SA, Qureshi AI. Outcomes of thrombolytic treatment for acute ischemic stroke in dialysis-dependent patients in the United States. J Stroke Cerebrovasc Dis. 2013;22(8):e354-9. [ Links ]
141. Tian SL, Murphy M, Han QF, Lu XH, Wang T. Prevalence and risk factors for peripheral artery disease among patients on maintenance peritoneal dialysis. Blood Purif. 2010;30(1):50-5. [ Links ]
142. Kuang DW, Li CL, Kuok UI, Cheung K, Lio WI, Xin J. Prevalence and risk factors associated with peripheral artery disease in elderly patients undergoing peritoneal dialysis. Vasc Health Risk Manag. 2012;8:581-6. [ Links ]
143. Tian SL, Tian XK, Han QF, Wang T. Peripheral arterial disease predicts overall and cardiovascular mortality in peritoneal dialysis patients. Ren Fail. 2012;34(8):1010-4. [ Links ]
144. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463-654. [ Links ]
145. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(1):S1-75. [ Links ]
146. Huang WH, Chen YC, Hung CC, Huang JY, Lin JL, Yang CW. Atherosclerotic risk factors among ankle-brachial index and toe-brachial index in peritoneal dialysis patients. Ren Fail. 2007;29(7):835-41. [ Links ]
147. Antithrombotic Trialists C. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71-86. [ Links ]
148. Lipscombe J, Jassal SV, Bailey S, Bargman JM, Vas S, Oreopoulos DG. Chiropody may prevent amputations in diabetic patients on peritoneal dialysis. Perit Dial Int. 2003;23(3):255-9. [ Links ]
149. Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, et al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46(5):897-902. [ Links ]
150. Abbott KC, Trespalacios FC, Taylor AJ, Agodoa LY. Atrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortality. BMC Nephrol. 2003;4:1. [ Links ]
151. Vazquez E, Sanchez-Perales C, Lozano C, Garcia-Cortes MJ, Borrego F, Guzman M, et al. Comparison of prognostic value of atrial fibrillation versus sinus rhythm in patients on long-term hemodialysis. Am J Cardiol. 2003;92(7):868-71. [ Links ]
152. Report UAD. Cardiovascular special studies http://www.usrds.org/2004/pdf/09_cardio_04.pdf; 2004. [ Links ]
153. Pun PH, Lehrich RW, Smith SR, Middleton JP. Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin J Am Soc Nephrol. 2007;2(3):491-500. [ Links ]
154. Tangri N, Shastri S, Tighiouart H, Beck GJ, Cheung AK, Eknoyan G, et al. beta-Blockers for prevention of sudden cardiac death in patients on hemodialysis: a propensity score analysis of the HEMO Study. Am J Kidney Dis. 2011;58(6):939-45. [ Links ]
155. Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005;68(2):818-25. [ Links ]
Ana Carina Ferreira, MD
Rua da Beneficência, nº8; 1069-166, Lisboa, Portugal
E-mail: a.carina.costa.ferreira@gmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: Mar 2, 2019
Accepted in revised form: Mar 8, 2019
ANNEX
Annex 1 – Peritoneal Dialysis Study Group of the Portuguese
Society of Nephrology:














