Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.34 no.2 Lisboa jun. 2020
https://doi.org/10.32932/pjnh.2020.07.079
TUBULAR QUIZ
A case of extreme alkalosis - the perfect combination of perpetuators
Patrícia Valério1, Hugo Ferreira2, Teresa Chuva2, Ana Paiva2, Inês Neves3, Filomena Faria3, José Maximino2
1 Nephrology Department, Centro Hospitalar de Setúbal
2 Nephrology Department, Instituto Português de Oncologia do Porto
3 Intensive Unit Care, Instituto Português de Oncologia do Porto, Portugal
CASE PRESENTATION
A 70‑year‑old Caucasian male, with an ECOG1 performance status of 1, was admitted in August 2019 for an elective surgery due to a stage II bladder urothelial carcinoma (pT2N0M0). The diagnosis was made one year ago and the patient had already undergone transurethral resection of bladder carcinoma six months earlier.
His past medical history consisted of hypertension, gout, smoking (50 pack‑year),cystinuria with cystin coralliform nephrolithiasis and chronic kidney disease (CKD) G3A1 [basal serum creatinine (Cr) of 1.5 mg/dL] with a right kidney atrophy. He was medicated with amlodipine/valsartan 5/160 mg id and alopurinol 300 mg id.
A radical cystectomy with total right nephroureterectomy and cutaneous left ureterostomy was performed on august 23rd, without complication on the immediate postoperative period. He started oral diet in the first 24 hours. Diuresis was preserved, about 100 to 125 mL/hour.
Three days later the patient presented with abdominal distention and gastric stasis. He remained fasting, with gastric tube draining freely.
Surgical team performed an exploratory laparotomy, with identification of an internal hernia of the small bowl, which was corrected.
In the postoperative period, nephrology collaboration was requested due to an acute kidney injury (AKI), with Cr of 3.39 mg/dL and urea of 89.5 mg/dL. On our observation, the patient presented an auricular temperature of 37.4°, adequate peripheral oxygen saturation (99‑100%) with oxygen supply by face mask (FiO2 28%) and a hypotensive profile (blood pressure of 114/66 mm Hg). The patient maintained passive drainage through nasogastric tube in the previous 3 days, around 3 to 3.5 L/day, without intestinal transit. Diuresis was preserved, about 60 mL/hour. He was prostrated and bradypneic, with mucosa severely dehydrated. The patient did not present any focal deficit and he answered simple questions only when vigorously stimulated. There was no alteration in pulmonary and cardiac auscultation. He was medicated with furosemide 20 mg bid and still on his anti‑hypertensive drugs. Empirical antibiotics (piperacillin/tazobactam) were started the day before.
Arterial blood gas analysis presented a severe metabolic alkalosis, with pH of 7,63, PaO2 of 90 mm Hg, PaCO2 of 99 mm Hg, bicarbonate of 104,1 mmol/L, sodium of 142 mEq/L, hypokalemia of 2.7 mEq/L, low ionized calcium of 0.86 mmol/L and lactate of 1.9 mg/dL. Blood work showed a Cr of 4,15 mg/dL, urea of 103,9 mg/dL, hypernatremia of 147 mEq/L, hypochloremia of 78 mEq/L, hypokalemia of 3.2 mEq/L, uric acid of 16.8 mg/dL, albumin of 3.2 g/dL, total corrected calcium of 9.5 mg/dL, elevated C‑reactive protein of 38.38 mg/dL and procalcitonin of 1.31 ng/mL. Urinalysis revealed an alkaline urine (pH 9.0), with 100 mg/dL of proteins and a urine microscopy with microhematuria (2‑5 red blood cells/high power field). Table 1 shows the relevant laboratory findings during hospital admission and follow‑up.
QUESTIONS
What is the cause of patient's metabolic alkalosis?
How can we explain the electrolytic changes?
How should we manage this patient?
ANSWERS
What is the cause of patient's metabolic alkalosis?
Metabolic alkalosis results from retention of alkali excess and it is characterized by an elevation of plasma bicarbonate concentration and, consequently, arterial blood pH.1 Normally, a physiological response leads to hypoventilation with a secondary increase of PaCO2.2
Plasma bicarbonate concentration can increase mainly due to excess hydrogen loss (renal or gastrointestinal), hydrogen movement into the cells and bicarbonate salts administration.3
The most common cause of metabolic alkalosis is hydrogen loss. In both renal or gastrointestinal losses, they are usually accompanied by the loss of chloride and potassium, resulting in hypochloremia and hypokalemia.1,3
Our patient maintained passive drainage through nasogastric tube in the previous 3 days, above 3 L/day, leading to a severe extrarenal loss of chloride and hydrogen, and a consequent bicarbonate increase.
This was made evident by urinary spot chloride (<20 mEq/L), despite the concomitant use of diuretics. The loop diuretic may have played a role, not only by causing alkalosis by itself, but also by worsening the process through renal losses of chloride, potassium and volume depletion.
In individuals with adequate kidney function, kidneys respond to alkalosis with an increase in alkali excretion1 through 3 mechanisms: increase in filtered load, allowing a higher amount of bicarbonate to reach the distal nephron; chloride‑dependent secretion of bicarbonate in the collecting duct (β‑intercalated cells), by pendrin (a Cl-HCO3‑ exchanger); and down‑regulation of hydrogen secretion in the collecting duct (inhibition of K+/H+‑ATPase in the α‑intercalated cells).1,4 Table 2 shows metabolic alkalosis classification according to pathophysiology.
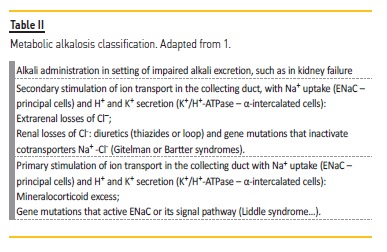
A sustained metabolic alkalosis only occurs when an additional factor impairs bicarbonate excretion or enhance its reabsorption, namely an impaired kidney function (in our patient, CKD with superimposed pre‑renal AKI); hypochloremia (large amount of chloride rich gastric fluid losses); volume contraction (our patient was severely dehydrated from nasogastric tube, improper fluid administration and forced diuresis with furosemide); hypokalemia (fasting, secondary hyperaldosteronism due to volume contraction and furosemide) and increased sodium delivery to the distal nephron that led to hydrogen and potassium secretion (a loop diuretic effect, but less important in our patient).1,3
Our patient presented all these factors, that impaired the above‑mentioned mechanisms, perpetuating alkalosis.
Hypoventilation and consequent PaCO2 elevation are a physiologic response to alkalemia, preventing life‑incompatible pH elevations.
For every 1.0 mM increase in arterial bicarbonate concentration, PaCO2 should increase 0.5 to 0.7 mm Hg.2 Mortality correlates with elevation of arterial pH: mortality rate could reach 45% in patients with arterial blood pH of 7.55 and 80% when pH is greater than 7.65. The prognosis is worse in patients with respiratory and metabolic alkalosis.5
The PaCO2 increase was lifesaving in our patient and it was appropriate to the degree of bicarbonate elevation (calculated respiratory compensation: PaCO2 96 mm Hg). Such a dramatic increase was allowed by an appropriate arterial PaO2 maintained by external oxygen supply, despite bradypnea - hypoxia would have limited hypercapnia.
How can we explain the electrolytic changes?
Our patient presented with severe hypochloremia, hypokalemia, hypernatremia and low ionized calcium.
The nasogastric tube was draining for several days, leading to a great loss of chloride and hydrogen rich fluid, provoking severe hypochloremia. Chloride depletion impairs chloride‑dependent bicarbonate excretion and concomitant potassium depletion in distal nephron, through activation of ROMK channel (principal cells). These mechanisms lead to low urinary chloride and elevated urinary potassium, which can be measured in a spot urine. This secondary potassium depletion plays an essential role in alkalosis perpetuation, through maintenance of chloride depletion [downregulating Na+‑K+‑2Cl‑ cotransporter (loop of Henle) and Na+‑Cl‑ cotransporter (distal tubule)] and through loss of hydrogen (stimulating distal nephron K+/H+‑ATPase in the α‑intercalated cells) in order to maintain electroneutrality. Furthermore, potassium depletion induces renal ammonia production, allowing acid excretion and sustaining metabolic alkalosis.1, 4 In our patient, chloride depletion, as well as volume depletion, was also exacerbated by furosemide. Further renal chloride losses can ensue when potassium depletion is severe and consequently much larger amounts of chloride are needed to correct alkalosis.
In this patient, hypernatremia is clearly hypovolemic, caused by severe dehydration due to nasogastric tube, poor fluid intake and forced diuresis. Hyperuricemia is also concordant with dehydration along with reduced glomerular filtration rate and furosemide use.6
The patient presented with hypocalcemia highlighted by low ionized calcium, despite normal corrected total calcium. This discrepancy is explained by alkalemia, which can alter the equilibrium of the albumin‑calcium complex. PH elevation enhance their binding, leading to a lower ionized calcium (but not total calcium). When major shifts in pH are present, it is prudent to directly measure the ionized calcium level in order to determine the presence of hypocalcemia, its risk of complications and need for correction. However, it is expected that ionized calcium increases with alkalosis correction.7
How should we manage this patient?
The priority in this patient is to correct volume depletion with chloride rich fluids (such as 0,9% NaCl), along with potassium supplementation and prevention of continuing loss of chloride.3,8
Monitoring urinary chloride concentration can help guiding chloride replenishment and responsiveness of alkalosis to chloride administration. Calcium supplementation should be guided by possible occurrence of arrythmias or other complications, rather than trying to normalize it.9 Furosemide and hypotensive medication suspension is mandatory.
If nasogastric drainage must be continued, proper parenteral nutrition must be placed while correcting ongoing losses of fluids and electrolytes. Losses of chloride and hydrogen could be reduced with inhibition of gastric acid secretion.6 Prokinetics may have a role to treat ileus or even surgical correction of mechanical bowel obstruction, if present.
To proper care and monitorization of this life‑threatening situation, the patient should be admitted to an intensive care unit (ICU). Due to severe AKI, dialysis with low bicarbonate dialysate may be indicated if the patient fails to improve with conservative measures or if life‑threatening complications do not allow time for conservative correction of alkalosis. Hydrochloric acid infusion may have a role in severe cases in which dialysis is contra‑indicated.3,8,10
Our patient was immediately admitted in ICU and he started on aggressive intravenous (IV) hydration with NaCl 0,9%, along with IV potassium supplementation and parenteral nutrition. Offending drugs where discontinued and a proton pump inhibitor was started. The patient evolved with respiratory failure, needing invasive mechanical ventilation. Twelve hours after ICU admission, arterial blood gas showed a pH of 7.60, PaCO2 of 60.0 mm Hg and bicarbonate of 58.9mmol/L, while maintaining reasonable urinary output. On the next day, due to persistence of ileus a new exploratory laparotomy was performed, with detection of dilation of the small intestine, but without identification of a mechanical obstruction. He was started on prokinetics and the ileus resolved after few days. The patient had a progressive improvement with supportive measures, with no needfor renal replacement therapy.
FOLLOW‑UP
He was discharged on September 19th completely recovered, with a Cr of 1.58 mg/dL, and his anti‑hypertensive medication was changed to captopril due to the etiology of the lithiasis. Three months after discharge his functional status was excellent (ECOG 1) and renal function was stable.
CONCLUSION
This is an extreme case of metabolic alkalosis, where a myriad of contributors gathered in a perfect storm to achieve a bicarbonate concentration above 100 mmol/L, thought to be incompatible with life and, to our knowledge, never reported in the literature. However, the pH value was maintained in life‑compatible values by an extreme respiratory compensation which may have saved the patient before treatment initiation. Despite the severity of the alkalosis and AKI there was no need for dialysis to adequately treat this patient. An approach correcting the cause and, at the same time, the perpetuators are the key factors to successfully treat a metabolic alkalosis.
References
1. Gennari FJ. Pathophysiology of metabolic alkalosis: a new classification based on the centrality of stimulated collecting duct ion transport. Am J Kidney Dis 2011;58(4):626‑636. [ Links ]
2. Adrogue HJ, Gennari FJ, Galla JH, Madias NE. Assessing acid-base disorders. Kidney International 2009;76:1239‑1247. [ Links ]
3. Galla JH. Metabolic alkalosis. J Am Soc Nephrol 2000;11:69‑375. [ Links ]
4. Hamm LL, Nakhoul N, Hering‑Smith KS. Acid‑base homeostasis. Clin J Am Soc Nephrol 2015;10:2232‑2242. [ Links ]
5. Anderson LE, Henrich WL. Alkalemia‑associated morbidity and mortality in medical and surgical patients. South Med J 1987;80(6):729‑733. [ Links ]
6. Gennari FJ, Weise WJ. Acid‑base disturbances in gastrointestinal disease. Clin J Am Soc Nephrol 2008;3:1861‑1868. [ Links ]
7. Dickerson RN, Alexander KH, Minard G, Croce MA, Brown RO. Accuracy of methods to estimate ionized and "corrected" serum calcium concentrations in critically ill multiple trauma patients receiving specialized nutrition support. J Parenter Enteral Nutr 2004;28(3):133‑141. [ Links ]
8. Gillion V et al. The patient with metabolic alkalosis. Acta Clin Belg 2019;74(1):34‑40. [ Links ]
9. Cooper MS, Gittoes MS. Diagnosis and management of hypocalcaemia. BMJ 2008;336:1298‑302. [ Links ]
10. Lisawat P, Gennari FJ. Approach to the hemodialysis patient with an abnormal serum bicarbonate concentration. Am J Kidney Dis 2014;64(1):151‑155. [ Links ]
Patrícia Valério, MD
Hospital de São Bernardo - Serviço de Nefrologia, Rua Camilo Castelo Branco
175, 2910‑549 Setúbal
E‑mail: p.valerios89@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: May 28, 2020
Accepted in revised form: Jun 29, 2020














