INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can cause inflammation and damage to any organ system. It is more prevalent in women than men across all age groups. Lupus nephritis (LN) is a major risk factor for morbidity and mortality in SLE and occurs in about 20-65% of patients, which may lead to end-stage renal disease (ESRD) in about 10% of those affected1. Male patients tend to have more frequent renal involvement and greater severity of disease2,3. The classic pattern of lupus nephritis in a kidney biopsy is an immune complex mediated glomerulonephritis. It has been found that ANCA positivity, more often antimyeloperoxidase (82%) than anti-proteinase 3 (7%), influences the histological pattern of lupus nephritis and is associated with worse baseline renal function and more active lupus serology4. The pathogenesis of ANCA in LN patients is still not clear. It was observed that, when ANCA is present, other autoantibodies appeared more frequently. This could be explained due to neutrophil extracellular trap (NET) formation (released by NETosis)5. These NETs could serve as a font of autoantigens that leads to the production of pathogenic antibodies, contributing to the progression of LN. Also, ANCA could play a role in the activation of the complement pathway in contributing to the pathogenesis of LN5. The presence of crescents, in diffuse proliferative lupus nephritis, has been linked with specific clinicopathological features, a poor treatment response and a worse kidney survival6. Therefore, the choice of treatment is crucial when trying to achieve complete remission, which is defined as urinary protein excretion <0.3 g/day, normal urinary sediment, and normal or stable renal function (within 10% of normal eGFR if previously abnormal renal function), in these severe cases7.
CLINICAL CASE DESCRIPTION
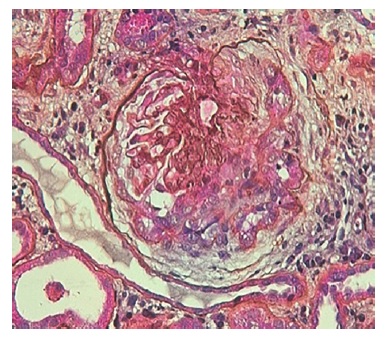
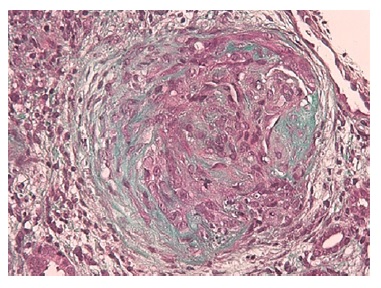
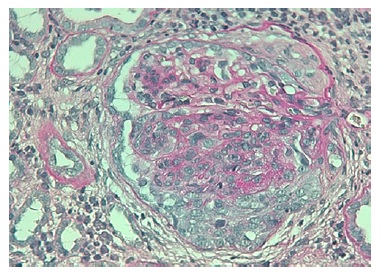
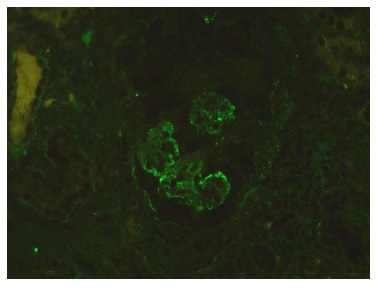
The authors present the case of a 19-year-old Caucasian male, without any relevant past medical history, who presented to the emergency department complaining of severe headaches for 5 days. The physical examination revealed peripheral edema and grade 2 hypertension (167/72 mmHg). The blood analysis revealed a normocytic normochromic anemia (Hg 7.6 g/dL; MCV 75 fL; MCHC 35g/dL ), with Cr of 2.64 mg/dL and BUN of 101 mg/dL, an acute kidney injury Stage II KDIGO: Kidney Disease: Improving Global Outcomes, and creatinine kinase of 1024 U/L. Hematuria (++) and proteinuria (100mg/dL) were also present in type II urinalysis. The cranial computerized tomography was normal. The patient was admitted to a general ward for treatment and etiological investigation. The investigation was remarkable for antibodies positivity for antinuclear (ANA), ANCA-proteinase 3, antidouble stranded DNA (Anti-dsDNA), anti-histone, anti-Smith, proliferating cell nuclear antigen (anti-PCNA) as well as low C3, C4 and CH50 levels (Table 1). The 24-hour urine analysis revealed proteinuria in the nephrotic range (6.9g/24h). A renal biopsy (Fig.1, 2, 3,4) was then performed. There were 15 glomeruli in the fragment and 10 of them presented medium to large crescents, ranging from cellular to fibrotic, adherent to Bowman’s capsule. Eight of these glomeruli presented with rupture of Bowman’s capsule and 6 presented fibrinoid necrosis and presence of karyorrhexis. Immunofluorescence was positive for IgG and C3 (Fig. 4). These deposits were of granular type along the glomerular wall in a segmental way. C1q deposits were not evident. Lack of glomeruli in the frozen segment meant immunofluorescence was performed in the paraffin fragment. This technique is less sensitive and therefore false negatives may exist. Electron microscopy was not performed. The patient was diagnosed with class IV Lupus Nephritis with modified NIH (National Institute of Health) Activity Index Score of 22 and Modified NIH Chronicity Index of 0. The initiation treatment of choice consisted of intravenous cyclophosphamide 1.0g/m2 (CIPH), monthly during 6 consecutive months, in association with prednisolone (1mg/kg/day) with slow tapering. The patient was discharged and was closely monitored via regular medical appointments. At 6-month follow-up CIPH was stopped and mycophenolate mofetil (MMF) (1g twice daily) initiated, as maintenance therapy, combined with prednisolone.
Table 1 Principal variables response to the chosen therapeutic strategy at follow-up
| Variable | Normal range | Admission | 6-month | 12-month | 18-month |
| Creatinine (mg/dL) | 0.7-1.2 | 2.64 | 1.6 | 1.4 | 1.1 |
| Total protein (mg/24h) | < 0.15g | 6800 | 600 | 250 | 100 |
| Ac. Anti-dsDNA IgG (IU/mL) | > 50 | 304 | 25 | 20 | 26 |
| Ac. Anti-C1q (U/ml) | <20 | 26.6 | 19.7 | 8.3 | 12.4 |
| C3 (mg/dL) | 90-180 | 68 | 78 | 80 | 82 |
| C4 (mg/dL) | 10-40 | 3,5 | |||
| CH50 | 42-95 | 32% | |||
| Ac. Anti-Proteinase 3 (U/mL) | <8 | 23 | 6 | 4 | 4 |
Although there were some signs of disease activity, such as microscopic hematuria, and C3 consumption were still present (a new biopsy was pondered but the patient refused any further invasive diagnostic measures), C1q levels decreased from 26.6 to 19.3 U/ml (negative if < 20 U/ml). ANCA-proteinase 3 and anti-dsDNA were negative.
The degree of proteinuria progressively declined and reached <300mg/day eight months after treatment onset. The creatinine values had a slow descent, reaching normal levels (1.2 mg/dL) after eighteen months of treatment (shown in Table 1). During this period the patient maintained MMF and prednisolone was gradually reduced to 5 mg and then suspended.
DISCUSSION
Cyclophosphamide (0.5-1 g/m2, monthly dose for 6 months, (also known as National Institute of Health (NIH) protocol) was the first immunosuppressive treatment that proved superior to a single-corticosteroids strategy8-11when treating LN. A lower-dose strategy (500mg iv every 2 weeks for 3 months), also known as Euro-Lupus regimen, demonstrated a similar efficacy to the previously stated regimen12,13; however, there was a low number of patients in the trial with widespread (>50%) segmental glomerular necrosis or crescents14. In some cases, MMF has been found to have an equal response rate to CIPH at 6 months, with no major differences in adverse effects15. The Kidney Disease: Improving Global Outcomes (KDIGO), the American College of Rheumatology Guidelines for Screening, Case Definition, Treatment and Management of Lupus Nephritis and the Joint European League
Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) guidelines all recommend for the treatment of Class III and IV or proliferative lupus nephritis an initiation therapy which include glucocorticoids associated with either CIPH or MMF14,16,17. The chosen strategy will depend on several factors such as race, disease activity and personal preference. MMF may be preferred in young men and women due to high risk of testicular and ovarian failure following treatment with cyclophosphamide18.
A study has found that patients with antineutrophil cytoplasmic antibody positivity serology were more likely to receive cyclophosphamide as treatment for their more active LN4. Also, in patients that present crescents on renal biopsy, an indication of severe LN, cyclophosphamide may be preferred, as it is one of the few drugs that has proven to be effective19,20. For maintenance therapy, the KDIGO guideline recommendations state that patients with class III and IV LN receive maintenance therapy with azathioprine (1.5-2.5mg/kg/d) or MMF (1-2 g/d in divided doses), and low-dose oral corticosteroids (<10 mg/d prednisone equivalent)14. A study has shown that MMF is more effective than azathioprine in maintenance therapy for preventing relapse, with no difference in clinically important side effects21. A regular evaluation should be performed to evaluate the patient’s response to treatment.
CONCLUSION
The authors emphasize the importance of this LN class IV case due to the concomitant presence of ANCA-proteinase 3 and the weight of the histopathological pattern when choosing initiation therapy. Both CIPH and MMF, in combination with corticosteroid, are effective options for initial therapy. In this young patient we probably would have chosen MMF as first-line therapy because of the absence of crescents and vasculitis in the renal biopsy. Because of the patient’s particular histopathological pattern with the presence of ANCAProteinase 3, it was decided that a protocol containing cyclophosphamide for initial therapy would be the best option. For the maintenance therapy, the patient initiated MMF with an oral corticosteroid with slow tapering. It is crucial to maintain a regular evaluation to rapidly detect signs of kidney function deterioration that can lead us to a redirection in therapeutic strategy with or without performing a new kidney biopsy to guide further treatment. In this case, after 2.5 years of immunosuppressive therapy, kidney function, proteinuria and lupus activity had an important decrease without significant complications.