INTRODUCTION
Chronic kidney disease (CKD) diagnosis is increasingly common in the elderly.1,2,3
According to the Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines, CKD is defined as a glomerular filtration rate (GFR) less than 60 ml/min/1.73m2 for three months or longer.4 A study performed among 610 individuals older than 70 years demonstrated that half had a measured GFR less than 60 mL/min/1.73m2 and would therefore meet criteria for the diagnosis of CKD.5 On one hand, this could be explained by the increased prevalence of risk factors for CKD such as hypertension and diabetes with age.6 On the other hand, it is known that after 30 years, GFR decreases progressively as a consequence of renal cells senescence.7,8,9
Cellular senescence can result from progressive telomere shortening consequence of aging or as a response to various pathophysiologic stressors. The accumulation of senescente cells that can no longer replicate has been implicated in the development of kidney fibrosis by secretion of pro‑fibrotic and pro‑inflammatory mediators leading to nephrosclerosis, arteriolar hyalinosis, and thus fewer functional glomeruli available.9,10,11
Distinguishing between the patients who have age‑associated kidney changes from those who have superimposed CKD is challenging. However, it crucial to identify which elderly patients have progressive kidney disease and a higher probability of developing severe forms of CKD from those whose renal function remains stable over time. This study aims to characterize patients with CKD over 80 years followed at a nephrology center and identify predictors for progressive versus non progressive CKD.
METHODS
Retrospective data was collected on patients aged over 80 years old with CKD, followed regularly at a nephrology single‑center.
Exclusion criteria comprised CKD diagnosis for less than 5 years and the absence of at least an annual creatinine value between 1st January 2014 and 31st December 2019. Therefore, 101 elderly patients were included in the study.The data were collected from patients’ electronic files and includeddemographic information, kidney disease cause, and comorbid conditions such as hypertension (blood pression superior to 140/90 mmHg or use of any anti‑hypertensive drug), obesity (body mass index more than 30 kg/m2), ischemic cardiomyopathy, and diabetes mellitus. Other metrics were the presence of CKD complications such as anemia - defined as hemoglobin levels less than 12.0 g/dL in women and less than 13.0 g/ dL in men - or the need for epoetin supplementation; metabolic acidosis was defined as serum bicarbonate less than 22 mmol/L or bicarbonate suplementation; hyperphosphatemia was defined as phosphorus levels superior to 5.5 mg/dL or the use of phosphate binders. Intact parathyroid hormone (iPTH) levels, and the need for vitamin D analogs was also analyzed. Proteinuria was assessed in a spot urine sample by urine protein‑to‑creatinine ratio and considered significant when over 1 g/g.
CKD progression was assessed by the mean annual rate of GFR decline over at least five years. GFR was calculated with the CKD‑EPI formula. Patients with GFR declines equal to or higher than 5 mL/min/1.73 m2/year were considered as presenting progressive kidney disease, and patients with a GFR decline less than 5 mL/min/1.73m2/year were considered as presenting non‑progressive kidney disease.
Other endpoints of interest were progression to end‑stage kidney disease (ESKD) or death from any cause through 31st December 2019.
Statistical Analyses
Categorical variables were presented as frequencies and percentages and were compared with the use of Fisher’s exact test or the chi‑square test as appropriate. In continuous variables, normal distribution was checked using the Shapiro‑Wilk test or skewness and kurtosis. Since continuous variables presented skewed distributions, they were presented as medians and 25th and 75th percentile. The Mann‑Whitney test was used for univariable analysis. Binary logistic regression was performed in multivariable analysis, adjusted to risk factors for CKD progression (age, gender, diabetes mellitus, hypertension, obesity, ischemic heart disease, metabolic acidosis, and proteinuria). Kaplan-Meier estimates were used to evaluate survival curves between the progressive and non‑progressive groups. Multivariate Cox proportional hazards were used to determine risk factors for mortality and included patients’ comorbidities (age, gender, diabetes mellitus, ischemic cardiomyopathy, hypertension, proteinuria, obesity, metabolic acidosis, anemia, iPTH, and progressive CKD).
All reported p‑values are two‑tailed, with a p value less than 0.05 indicating statistical significance. Statistical analyses were performed by SPSS version 23.0 (SPSS Inc., Chicago, IL).
RESULTS
A total of 382 patients over 80 years were followed in ournephrology center. Of these, 101 were included in this study, and the follow‑up was 4.2 [1.7‑6.9] years.
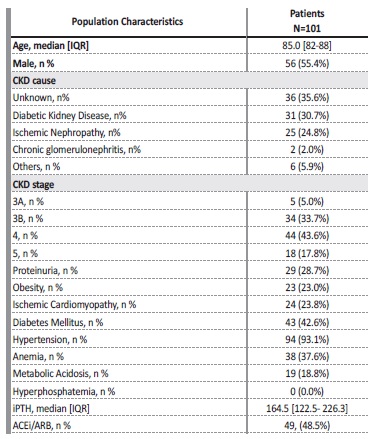
The clinical characteristics of the population are summarized in Table I. More than one‑third of patients presented CKD of unknown cause, and none of the patients had performed a kidney biopsy. Proteinuria over 1 g/g was present in 28.7% of patients, and 48.5% were using angiotensin converting enzyme inhibitors or angiotensin receptor blockers. There was a high prevalence of comorbidities: hypertension was present in 93.1%, diabetes mellitus in 42.6%, and ischemic cardiomyopathy in 23.8%. Regarding CKD complications, anemia was present in 37.6% of patients, and of these, 50.0% needed erythropoiesis‑stimulating agents. Metabolic acidosis was present in 18.8%. None of the patients presented hyperphosphatemia, and none were medicated with phosphate binders. Median iPTH levels were 164.5 [122.5‑226.3].
ACEi/ARB - angiotensin converting enzyme inhibitor/angiotensin receptor blocker; CKD - Chronic
Kidney Disease; iPTH - intact parathyroid hormone; std - standard.
Vitamin D analogs were used on 5.0% of the patients. The median rate of decline of GFR was 3.0 [2.1‑6.0] ml/min/1.73m2/year.
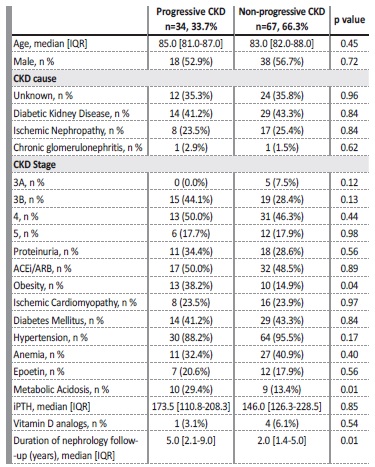
The comparison between the progressive and non‑progressive CKD groups is shown in Table II. The large majority (66.3%) presented non‑progressive kidney disease. Nephrology follow‑up time was also diferente between groups as progressive kidney disease group presented a longer follow‑up (5.0 vs 2.0, p=0.01). Concerning KDIGO CKD stages, there were no statistically significant differences between groups (Table II).
ACEi/ARB - angiotensin converting enzyme inhibitor/angiotensin receptor blocker; CKD - chronic
kidney disease, IQR - interquartile range, iPTH - intact parathyroid hormone
Compared to the non‑progressive CKD patients, the progressive kidney disease ones were obese (38.2% vs 14.9%, p=0.04) and presented more frequent metabolic acidosis (29.4% vs 13.4, p=0.01).
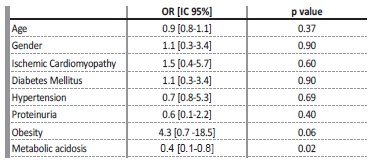
Proteinuria was slightly more common in the progressive group, but without statistical significance (34.4% vs 28.6%, p=0.56). After adjusting for other variables (age, gender, diabetes mellitus, hypertension, obesity, ischemic cardiomyopathy, metabolic acidosis, and proteinuria), only the presence of metabolic acidosis (OR: 0.4, CI: 0.1‑0.8)
was a predictor of progressive kidney disease (Table III).
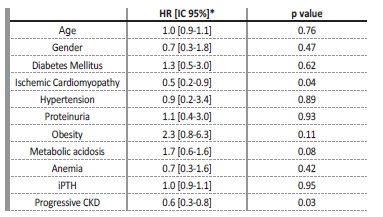
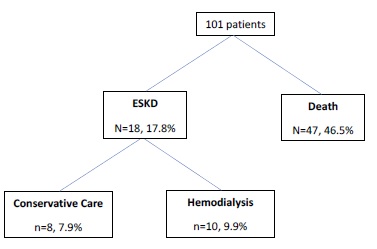
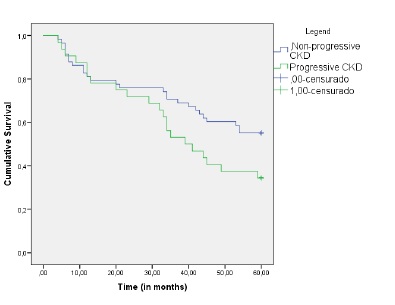
During the study follow‑up, 46.5% of patients died and 17.8% reached ESKD Figure 1. Of those, most patients (55.6%) initiated hemodialysis and the remaining were on conservative care. Figure 2 shows the Kaplan‑Meier curves to compare survival between progressive and non‑progressive CKD groups. The progressive CKD group had higher mortality (log rank 4.5, p=0.03). After adjusting for confounders, progressive kidney disease (OR 0.6, CI:0.3‑0.8) and ischemic cardiomyopathy (OR: 0.5, CI: 0.2‑0.9)
were risk factors for all‑cause mortality (Table IV).
*HR were adjusted for age, gender, diabetes mellitus, ischemic cardiomyopathy, hypertension, metabolic acidosis, anemia, iPTH, proteinuria, and obesity.
DISCUSSION
In our study, most of the very elderly patients presented non‑progressive CKD and CKD cause was unknown in more than one‑third. This is in line with other studies which show a slow GFR decline in the elderly.12,13,14
The slow GFR decline and the absence of an evident cause for CKD could be attributed to renal senescence, as has been reported.8,10Diabetic kidney disease is currently the leading cause of CKD in developed countries15 and is the second‑leading cause of CKD in this population. This could be a result of the competitive effects of death and progression to ESKD before the age of 80 years.
Our findings also suggest that metabolic acidosis is a predictor of progressive CKD. There is evidence that metabolic acidosis leads to CKD progression.16,17,19
A study with 134 patients with stage 4 CKD and metabolic acidosis randomized to either usual care or replacement with oral sodium bicarbonate for 2 years showed that patients receivingthe supplementation had a significantly slower progression of CKD.18
A sub‑analysis of the AASK trial suggested that serum bicarbonate level is an independent predictor of CKD progression and that higher serum bicarbonate levels within the normal range are associated with reduced hazard of mortality and CKD progression.19 In the kidney, metabolic acidosis is associated with an increase in aldosterone, endothelin, and angiotensin II levels.20‑23Endothelin‑1 mediates increased renal acid excretion in response to a systemic acid challenge and pharmacological inhibition of endotelin‑1 receptors using bosentan inhibited distal tubule acidification.21,22 Renin‑angiotensin system activation is important for urinary acidification and also promotes proteinuria, kidney damage, and progressive CKD.23 Metabolic acidosis is also linked to protein‑energy malnutrition and inflammation.24,25
Unfortunately, data regarding nutritional and inflammatory status was not available and therefore was not included on this study.
Proteinuria, is one of the most established factors for CKD progression26,27, was not statistically different between groups. We recognize that the use of the urine protein‑to‑creatinine ratio for proteinuria assessment in an elderly population with reduced muscle mass and common asymptomatic bacteriuria could induce some error.
Relative to other CKD complications, both groups were similar in terms of anemia, epoetin supplementation, and secondary hyperparathyroidism. It is relevant that none of the patients presented hyperphosphatemia. This could be caused by the decreased tubular reabsorption of phosphorus7 that occurs in elderly people, associated with the low dietary intake that is common in this population28.
Nephrology follow‑up was longer in the progressive group. This is probably explained by the discharge of some patients with stable GFR, as opposed to patients with progressive GFR who are more likely to maintain nephrology follow‑up.
Also, patients with a faster GFR decline are more likely to be referenced to nephrology follow‑up earlier.
During the study follow‑up, considerably more patients died than progressed to ESKD. This is in line with other studies that reported that patients over 75 years old are more likely to die prior to progression to renal replacement terapies.29‑ 31
In our cohort, the risk of death was superior in the progressive group and the risk of all‑cause mortality was higher in patients with progressive CKD and in patients with
ischemic cardiomyopathy. Therefore, progressive CKD in the elderly is likely to represent a pathological process and therefore it is a marker of multiple comorbidities and thus mortality.30
This study has some limitations that should be pointed out. First, it is a single‑center
retrospective study with a relatively small population and with the already known limitations related to the use of creatinine‑based formulas to calculate GFR and protein‑to‑creatinine ratio to assess proteinuria in the elderly.
Our results showed that most elderly patients have non‑progressive chronic kidney disease, and that metabolic acidosis seems to be a predictor of progressive disease. Therefore, metabolic acidosis, something that is sometimes overlooked when assessing CKD comorbidities, should be carefully assessed in elderly patients. Since metabolic acidosis is linked to nutrition and inflammation, more studies are needed to address the impact of these factors on CKD progression.
Given that most patients died prior to progressing to ESKD, it is important to look at CKD in the elderly as an important marker of comorbidity. Beyond progressive CKD, ischemic cardiomyopathy is na important risk factor for all‑cause mortality. These results emphasize the importance of the implementation of cardiovascular protection measures in those patients.