INTRODUCTION
Vascular access in hemodialysis (HD) is a sine qua non to treat patients, representing a lifeline. There are three main types of vascular access in hemodialysis - arteriovenous fistulas (AVFs), arteriovenous grafts (AVGs), and central venous catheters (CVCs). Compared to AVGs and CVCs, AVFs have lower complication and reintervention rates and longer cumulative patency. In addition, patients with AVFs have lower access-related hospitalization and mortality rates1,2,3. For these reasons, AVFs are deemed as the first-choice access for most patients who have suitable vessels and a long life-expectancy4. However, decisions regarding vascular access creation should always take into consideration the patient’s overall End Stage Kidney Disease (ESKD) Life-Plan, which is a personalized strategy for living with ESKD, encompassing vascular access planning, ideally made together and periodically reviewed by the patient and a coordinated chronic kidney disease (CKD) management team. Attainment of the “right access, in the right patient, at the right time, for the right reasons” contrasts with “fistula first” policies as a more patient-centered approach to care4,5.
AVFs result from an anastomosis between an artery and a vein, which can be created by open surgery or, more recently, percutaneous endovascular techniques. Once created, the arterialized vein goes through a maturation process where it acquires the necessary diameter and flow to be cannulated repeatedly, safely, providing an adequate HD treatment. Provided that all goes well, this process usually lasts 4 to 6 weeks6. However, 20 to 60% of newly created AVFs do not mature adequately to be used in HD - primary failure rate or non-maturation rate7,8,9,10. This largely relates to stenoses adjacent to the anastomosis or in the outflow tract and can be corrected by open surgical or percutaneous endovascular rescue techniques if identified in time11. Furthermore,
excluding AVFs with primary failure, only 70% of AVFs are patent after one year without being submitted to an intervention to assist patency - primary patency or unassisted primary patency rate7.
This article reviews and summarizes the existing literature, including recent guidelines, on early identification, treatment, and follow-up of immature AVFs, offering a practical approach to reducing primary failure rates, thrombosis, and to improving their overall longevity.
AVF MATURATION
Once an AVF is created, there is a reduction in vascular resistance which generates increased blood flow. The increased flow in turn results in augmented wall shear and circumferential stress, the hemodynamic forces driving both artery and vein remodeling. The endpoint of this process is referred to as AVF maturation, which can be characterized physiologically using criteria related to blood flow and vessel diameter12
(Table I). Almost fifty percent of the AVF total flow increase was found to take place on the first day after the procedure13 and full maturation in uneventful new AVFs is usually accomplished between the second and fourth week14 - as such, cannulation is preferable after the fourth week15. However, if it will avoid placement of a central venous catheter, cannulation might be performed after the second week15,16without increasing the risk of AVF failure, according to an analysis from the DOPPS17. From the same study, only AVFs cannulated within 14 days were at increased risk of subsequent failure (2.1-fold)17.
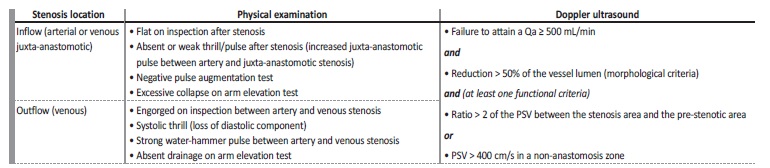
Physical examination by experienced staff may predict maturation in up to 80% of AVFs14,18. Equally, physical examination has great accuracy detecting underlying culprit lesions for non-maturation, such as inflow or outflow stenosis19. Nonetheless, there is significant variability between dialysis staff and centers justifying the usual added use of objective and reproducible physical parameters (blood flow, vein diameter and depth) obtained by Doppler ultrasound, which is an accurate, noninvasive, inexpensive and diagnostic test (Table I). A 2002 University of Alabama study proposed a combination of blood flow ≥ 500mL/min and vein diameter ≥ 4mm for AVF maturation criteria, generally present after a month, which has a positive predictive value of 95% for adequate AVF utilization and, hence, maturation14 (Table I). Subsequently, in 2006, the Kidney Diseases Outcomes Quality Initiative (KDOQI) clinical practice guideline proposed, based on expert opinions, a combination of blood flow ≥ 600 ml/min, vein diameter ≥ 6 mm and depth from the skin ≤ 6 mm - “rule of 6”20 (Table I). More recently, in a study of CKD patients undergoing AVF creation, blood flow, vein diameter, and depth obtained by Doppler ultrasound, moderately predicted the likelihood of AVF maturation at six weeks21.
Primary failure rates due to non-maturation has increased over the years, which is probably a result of demographic changes in hemodialysis patients from young to elderly, comorbid patients with cardiovascular disease, and diabetes mellitus as a major cause for renal failure7. This shift translates into calcified, noncompliant arteries, and sclerotic veins, resulting in inappropriate vascular remodeling, intimal hyperplasia, and failure to mature22. However, nearly all immature AVFs have a treatable underlying lesion, mostly stenosis, that can generally be identified and salvaged by surgical or endovascular techniques23,24,25.
According to one study, 80% of these AVFs could be rescued, thereby producing an increase in the likelihood of maturation by approximately 50% comparing to immature AVFs not submitted to these treatments25.
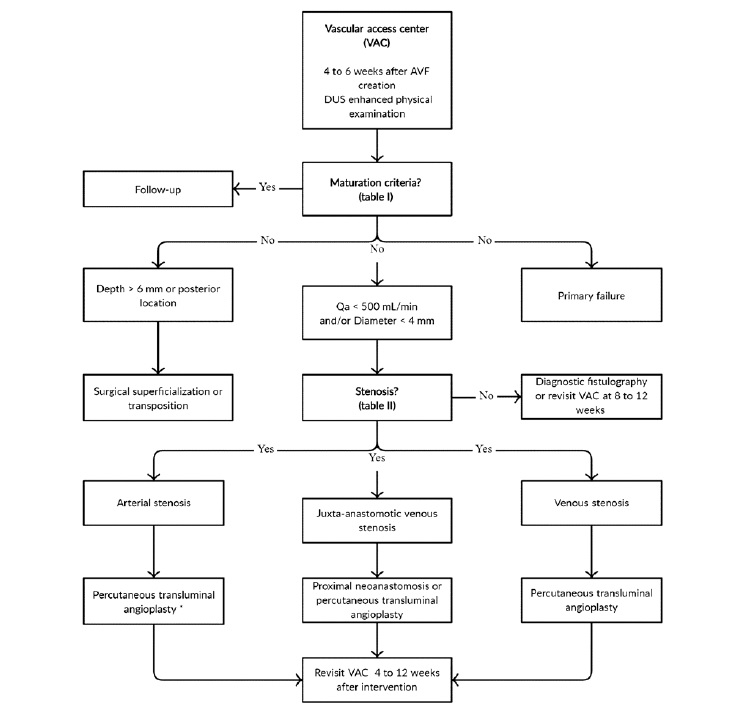
For these reasons, new AVFs maturation criteria should be assessed by physical and Doppler ultrasound examination 4 to 6 weeks after their creation, to identify immature AVFs’ underlying problems and consequently program elective treatment15,16,26.
According to our review of the literature, we propose a synthesized approach algorithm for newly created AVFs (see Figure 1). There are two degrees of AVF immaturity - physiological and/or clinical. Na AVF is considered physiologically immature if it has low blood flow and/or a small vessel diameter, as measured by Doppler ultrasound.
This is generally related to stenotic phenomena, which can be perceived by physical examination and/or Doppler ultrasound (Table II ). Additionally, and despite physiological maturity, an AVF can only be considered fully mature if it is clinically accessible for safe, repeated cannulation, according to depth, location on patient’s arm, and/or length. Deep (e.g. arm brachiobasilic AVF or obese patients) or posterior AVFs (e.g. forearm ulnar-basilic AVF) should be considered for surgical superficialization or transposition, whilst AVF stenosis compromising physiological maturity is an indication for surgical or endovascular treatment, depending on its location and available local resources4,15,16.
PSV - peak systolic velocity; Qa - blood flow
AVF - arteriovenous fistula; DUS - doppler ultrasound; Qa - blood flow. Primary failure - deemed surgically or endovascularly unsalvageable by the vascular access center team).
* Assuming no risk of provoking steal syndrome (hand hypoperfusion).
IMMATURE AVFS RESCUE TECHNIQUES
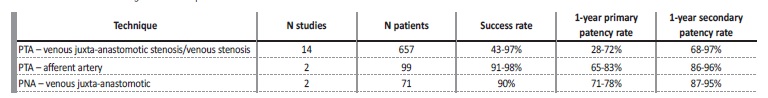
There are no randomized clinical trials comparing surgical to endovascular techniques on the rescue of immature AVFs. Stenoses located on the afferent artery or on a vein segment other than the juxta-anastomotic region are usually treated endovascularly by percutaneous transluminal angioplasty (PTA), given its minimally invasive nature and safety11,16. Outcomes with PTA on aferente artery stenosis look particularly favorable, comparing to other locations (Table III)27. As for juxta-anastomotic venous stenosis (≤ 5 cm from anastomosis), a narrative review of 28 non-randomized studies suggests a better primary patency rate with surgical revision, particularly with proximal neoanastomosis (PNA)11 (Table III). A retrospective study comparing PNA to PTA in immature radialcephalic AVFs juxta-anastomotic stenosis showed a better primary patency rate with the former (71% vs 41%), despite similar complication and secondary patency rates28. This data could point to surgical revision as the first line option for immature AVFs with juxta-anastomotic stenosis16- however, due to the lack of randomized clinical trials, this is still not consensual. According to the 2019 guidelines from the European Renal Best Practice and KDOQI, there’s insufficient evidence to support one over the other4,15(Table IV).
PTA - percutaneous transluminal angioplasty; PNA - proximal neoanastomosis
Meanwhile, the role of surgical or endovascular exclusion of accessory veins when rescuing immature AVFs remains to be elucidated, since these almost always coexist with stenosis, which should be treated first11,29. However, if this is not the case and manual compression of the accessory vein translates into enhanced blood flow through the AVF, it could be worthwhile to ligate surgically or endovascularly30. On the same note, there is currently insufficient data to recommend intraoperative maturation enhancement techniques (type of anesthesia, surgical technique, suture technique, anastomotic devices, intra-operatory angioplasty followed by balloon-assisted maturation), early (< 6 weeks) protocol-driven sequential balloon-assisted maturation or pharmacological interventions to improve AVF maturation rates4.
MONITORING AFTER MATURATION
AVFs submitted to more than one rescue intervention before maturation have a higher risk of thrombosis and permanent loss than AVFs that mature with one or no interventions. Furthermore, in these cases, more interventions are required to maintain long-term patency31,32. Follow-up surveillance programs of mature AVFs operate under the assumption that early identification and preemptive treatment of stenosis may prevent thrombosis and permanent access loss. Clinical monitoring through physical examination of the AVF by experienced staff has high sensitivity and specificity for the detection of AVF stenosis19. However, given AVF Qa-based surveillance’s possible
added benefit on the early identification of stenosis, reduction of thrombosis, and permanent access loss33,34,35.36, periodic assessment by Doppler ultrasound in addition to clinical monitoring could be considered (especially, but not exclusively) on AVFs submitted to rescue interventions before maturation, due to their higher risk of thrombosis and permanent access loss. However, formal evidence-based recommendations for the latter strategy over clinical monitoring alone are lacking due to insufficient data4,15.
Multidisciplinary care
To start and sustain HD with an adequately mature AVF depends on the work of multiple professionals - nephrologists, vascular surgeons, interventionalists, and nurses. In addition to an adequate referral timing for AVF construction, vascular mapping, and surgical experience, a good outcome is best attained by implementing protocolized programs for AVF follow-up, involving multidisciplinary participation16.
These programs are probably most effective if there is: 1) consensos regarding procedures to identify AVF non-maturation, underlying among all parties involved; 3) a dedicated vascular access coordinator who acts as a liaison between all services; 4) prospective tracking of outcomes with continuous quality assessment and improvement37.
In Spain, the replacement of an isolated vascular surgery medical appointment by a joint nephrology and vascular surgery appointment with Doppler ultrasound and objective criteria for radial-cephalic AVF creation and AVF elective treatment resulted in an improvement of the primary failure rates38.
CONCLUSION
AVFs are first-choice vascular access for most HD patients. Some AVFs do not mature adequately, mostly due to stenosis. Surgical and endovascular techniques can rescue an important percentage of these AVFs from primary failure, allowing them to be safely and effectively used in HD, preserving venous capital and avoiding the placement of CVCs and associated complications. Hospitals and HD clinics should aim to systematically evaluate AVFs four to six weeks after surgery to facilitate timely diagnosis of AVF non-maturation and subsequente orientation for elective treatment according to the underlying cause.
These AVFs should then be periodically assessed for dysfunction to preemptively correct any reoccurring stenoses, ideally through multidisciplinar protocolized follow-up programs.