INTRODUCTION
Cardiovascular disease (CVD) continues to be the most frequente cause of death in dialysis patients.1‑3Whichever the dialysis modality, heart failure (HF) and CVD need an improved approach that is currently limited by the few studies on this population. In Portugal, CVD was responsible for 41.5% of PD deaths in 2019.3 CVD manifests itself as acute myocardial infarction, cerebrovascular events, arrhythmia, HF and sudden cardiac death. HF especially represents a high burden for PD patients since it is responsible for a high number of hospitalizations. In a 4‑year follow‑up study with 220 PD patients, 28% developed new‑onset HF and 62% of those with previous HF had an episode of worsening HF requiring hospitalization.
The cumulative 4‑year survival probability was 37.4% and 64.7% for patients with and without previous HF, respectively.4 The major phenotype of HF on PD is diastolic dysfunction and marked left ventricular hypertrophy (LVH).4‑6
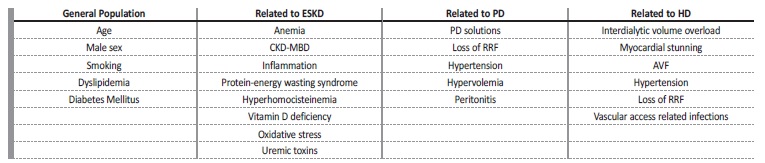
Risk factors for HF in these patients include those that affect the general population as well as those related to end‑stage kidney disease (ESKD) and those that are specific to PD.7‑10PD has also some potential cardiovascular advantages, in particular the avoidance of the unphysiological fluctuations in fluid and solute status associated with hemodialysis. Unquestionably, the longevity of patients on PD is related to improvements in the diagnosis, management, and prevention of CVD and HF, but little is known about the physiopathology of both, on PD patients. In this review, we will explore the spectrum of HF on PD patients, its pathogenesis, risk factors and possible therapeutic and preventive measures.
HEART FAILURE ON PERITONEAL DIALYSIS
HF diagnosis is challenging in PD patients. Echocardiography, the most used method for left ventricle evaluation, is volume‑dependent and parameters such as elevated left ventricular end‑diastolic pressure, increased estimated pulmonary artery systolic pressure or left ventricular ejection fraction may be altered on patients with hypervolemia.11 International Society for Peritoneal Dialysis (ISPD) guidelines suggest an echocardiography evaluation after PD initiation for assessment of LVH and systolic and diastolic function as well as cardiac valvular abnormalities including valvular calcification.12 A reassessment should be carried out whenever clinical status change. Likewise, N terminal pro‑brain natriuretic peptide (NT‑proBNP) is an importante HF biomarker and its levels have been found to be higher in patients on dialysis than in the general population.13,14NT‑proBNP elevation in PD patients is an important predictor of cardiovascular congestion, mortality, and adverse cardiovascular outcomes and seems to add important prognostic information beyond that provided by LVH, systolic dysfunction, and other conventional risk factors.13,14
Based on left ventricle function, HF is classified into two major types with different physiopathology and treatment options: heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF).15 The main phenotype on PD patients is HFpEF with diastolic dysfunction and marked LVH, also known as uremic cardiomyopathy.4‑6
Heart failure with preserved ejection fraction - Uremic cardiomyopathy
Almost 70% of patients starting PD have detectable LVH by echocardiography and changes in cardiac structure tend to progress during dialysis treatment.16 LVH is the strongest independent predictor of cardiovascular mortality in patients with ESKD independently of other risk factors.10,16‑18
Diastolic dysfunction seems to be related to a decrease in the active reuptake of calcium into the sarcoplasmic reticulum as well as to ventricular fibrosis, which impairs passive relaxation and precedes LVH.6,19 The pathogenesis of uremic cardiomyopathy on dialysis patients seems to be multifactorial, and available evidence strongly suggests that inflammation, FGF23 and low Klotho levels affect cardiac remodeling and therefore are involved in the development of diastolic disfunction and LVH.6,20 Early diastolic disfunction is difficult to diagnosis but since it is high prevalent on PD patients, other echocardiographic techniques such as tissue doppler velocities should be considered in order to identify myocardial dysfunction at the preclinical stage and implement interventions to prevent its progression.21
Heart failure with reduced ejection fraction
ISPD guidelines recommend that PD patients who have a persistente reduction in ejection fraction of less than 40 percent should be further evaluated for coronary heart disease (CHD).12 The prevalence of CHD is much higher than that observed in the general population but may still be underdiagnosed since CHD often has an atypical presentation in PD patients.22 Also, clinically silent CHD is common among dialysis patients and conveys a similar negative prognosis compared with a clinically recognized myocardial infarction.22,23Its diagnosis remains challenging since noninvasive methods used for diagnosis of silent CHD in the general population such as troponin levels, electrocardiography, stress echocardiography and pharmacological perfusion imaging, lack accuracy for CHD surveillance among PD patients.12,22,23
General risk factors
PD patients share some risk factors for HF and CVD with the general population, such as age or male sex.8 (Table I) However, the effect of obesity on cardiovascular risk on PD patients is inconsistent. Some studies have found that obesity confers a survival advantage,24,25 whereas others have reported an association of obesity with na increased risk of mortality,26 or no association at all.8 Dyslipidemia is an established risk factor for CVD in the general population, but data is limited with regards to PD. In theory, PD provides an ideal milieu for worsening dyslipidemia, both by continuous glucose absorption and significant albumin loss, although evidence of such cause‑and‑effect link is sparse.27 Current guidelines do not recommend starting statins on patients already on dialysis, although a recente study on hemodialysis patients showed that statin therapy, preferably combined with ezetimibe, was associated with a lower risk of all‐cause mortality.27,28 Glycemic control, which is also an established risk factor for CVD in diabetics, is difficult in PD patients, due to the constant glucose absorption.2,7,9,29 In addition to glycemic control in diabetic patients, studies suggest that PD patients with morning blood sugar values between 100 and 126 mg/dl are at a higher risk of death compared to those with levels below 100 mg/dl.2 Therefore, periodic glycemic control is important even in non‑diabetic PD patients, and in individuals in whom glycemic values are elevated and insulin resistance is suspected, aggressive lifestyle interventions should be implemented.
Risk factors related to end‑stage kidney disease
Several potential factors have been investigated to explain the high prevalence of endothelial dysfunction and cardiovascular mortality among PD and hemodialysis patients(Table II). Hyperphosphatemia, hyperparathyroidism, and hypercalcemia (caused by high doses of vitamin D or calcium‑based phosphate binders) have been implicated in the calcification process which is a significant risk factor for hypertension, cardiac remodeling, LVH and cardiovascular may cause decreased diastolic filling, and increased ventricular afterload.30 Klotho seems to prevent the development of LVH via blocking the activation of the renin‑angiotensin‑aldosterone system (RAAS), the sympathetic nervous system and transforming growth factor‑β and could be a potential therapeutic target.2,6
Evidence suggests that 25‑hydroxyvitamin D levels are lower in PD patients, but whether supplementation could prevent cardiovascular events is not yet proved. Wang et al. confirmed that low sérum 25‑hydroxyvitamin D levels were associated with an increased risk of fatal or nonfatal cardiovascular events, but in that study the influence of vitamin D on cardiovascular outcomes seemed to closely relate to residual renal function (RRF), severity of LVH, and cardiac dysfunction.31 mortality in ESKD.2,6,9 Even without atherosclerosis, coronary medial calcification
Table II Interventions for improvement of cardiovascular and metabolic control of peritoneal dialysis patients
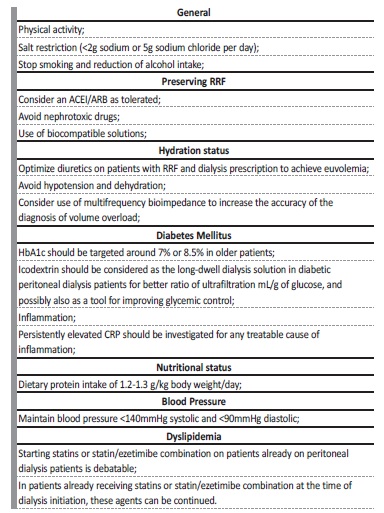
ACEI - angiotensin‑converting enzyme inhibitor; ARB - angiotensin‑receptor blocker; CRP - C Reactive Protein; RRF - residual renal function
Anemia is a significant predictor of death in patients undergoing PD.32 Potential mechanisms that may explain the relationship between anemia and the development of LVH include reduced oxygen delivery to the myocardium, increased cardiac output, increased oxidative stress and activation of the sympathetic nervous system.4,7 Also, erythropoietin may have direct effects on myocardial function, since the treatment of severe anemia with erythropoietin stimulating agentes is associated with improvement of LVH.33
Chronic inflammation, as demonstrated by an elevated C reactive protein (CRP), is associated with higher cardiovascular risk in PD patients.4,7 Since malnutrition, inflammation, and atherosclerotic CVD often coexist, these risk factors have been proposed to be pathophysiologically linked and are referred to as the MIA syndrome.34
RISK FACTORS RELATED TO PERITONEAL DIALYSIS
There are inherent factors in the PD technique that increase the risk of CVD. Comparison between continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis (APD) have been made in terms of cardiovascular risk, but the variability of prescriptions among studies makes impossible to draw conclusions.35,36 Therefore, based on current data, no modality should be preferred to the other based on the cardiovascular risk of the patient.
Dialysis Solution
The continuous exposure to dialysate glucose leads to the formation of advanced glycation end‑products that are associated with peritoneal membrane damage and loss of RRF.7 Advanced glycation end‑products could also have a role in the cardiovascular risk of PD patients, but randomized studies with both biocompatible and standard solutions have not shown better survival outcomes.37 Several studies have suggested clinical benefits of icodextrin solutions regarding fluid management and improved glycemic and metabolic control.38,39 It is also hypothesized, that the instillation of the dialysis solution into the peritoneal cavity could result in an elevation of systemic blood pressure due to a rise in total peripheral resistance even in the absence of hypervolemia.7
Loss of RRF is an important risk factor for all‑cause mortality and CVD among PD patients.7‑9,40,41 The CANUSA study revealed that each 5 L per week per 1.73 m2 increment corresponded to a 12% decrease in the relative risk of death.40 The ADEMEX study also corroborates this data.41 More recently, a prospective study by Wang et al. in 246 CAPD patients, 39% of which were completely anuric, demonstrated more adverse cardiovascular, inflammatory, nutritional, and metabolic profiles and a higher overall mortality in the anuric population when compared to those patients with a residual glomerular filtration rate superior to 1mL/min/1.73m2.42
Hypervolemia and hypertension
Hypervolemia is common in PD patients and is associated with development of endothelial disfunction, LVH and consequently poor outcomes.7‑9Since hypertension is a consequence of hypervolemia in most cases, targeting an adequate dry weight is also essential for blood pressure control. Hypertension is as well directly correlated with LVH and diastolic dysfunction and consequently an importante risk factor for HF.7‑9,43
Hypervolemia can occur due to loss of RRF, high sodium and water intake or due to ultrafiltration failure. PD prescriptions should also be tailored to optimize ultrafiltration and volemia. This includes, for example, the adjustment of the dwell time to the peritoneal transport status and the use of icodextrin for the long dwell.12 Various studies validate the impact of hypervolemia on cardiac function, highlighting the importance of a close monitorization of volume status of PD patients to prevent HF progression.8‑10,44
Multifrequency bioimpedance can be a useful tool to increase the accuracy of the diagnosis of volume overload. Despite its usefulness, bioimpedance still does not discriminate the amount of extracelular water that is in the intravascular compartment, available for ultrafiltration, and which is in the interstitial compartment and that should not be removed at a higher rate.45 HF patients have a narrow safety window for euvolemia, and a stepwise weight reduction is crucial to avoid end‑organ ischemia.
Peritonitis
Episodes of peritonitis have been shown to correlate with increased mortality. In a study of 1321 PD patients, peritonitis was independently associated with increased risks of all‑cause, cardiovascular, and infection‑related mortality.46 Furthermore, it has been shown that severe or repeated peritonitis may be associated with deterioration of peritoneal membrane status and UF capacity, leading to worse volume control.7 Also, a chronic inflammatory state may persist even after successful treatment of peritonitis which may predispose to enhanced cardiovascular risk.9
INTERVENTIONS FOR PREVENTION AND TREATMENT OF HEART FAILURE ON PD PATIENTS
Since CVD and particularly HF is responsible for high mortality and morbidity, it is crucial to implement measures to prevent the progression of HF on PD patients. Table II shows measures recommended by ISPD, KDIGO and ERBP guidelines for a better cardiovascular and metabolic control.12,27,47
HF treatment in PD patients is not well defined due to the shortage of studies in this population. Selected cases could be candidates for implantable cardioverter defibrillator (ICD) or nodal auricular ablation for atrial fibrillation.15,48Some data report a higher rate of peri‑procedure complications in dialysis patients. Furthermore, dialysis patients have historically been excluded from ICD trials, as the competing risks associated with renal failure were anticipated to diminish the benefits of device therapy.48 There are no guidelines for these procedures on dialysis patients, so decisions must be made on a case‑by‑case basis. In line with European Society of Cardiology (ESC) guidelines, HF treatment is different in HFpEF and HFrEF and is therefore presented in separate topics in this review.15
Treatment of heart failure with preserved ejection fraction/uremic cardiomiopathy
An important component of treating a patient with HFpEF is to treat the concomitant cardiovascular conditions, such as hypertension, CHD, atrial fibrillation, and non‑cardiovascular diseases such as chronic pulmonary disease, anemia, diabetes mellitus and sleep disorders.
Compared with HFrEF patients, hospitalizations and deaths in patients with HFpEF are more likely to be non‑cardiovascular.15 Trials of angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta‑blockers and mineral receptor antagonists (MRAs) have all failed to reduce mortality in patients with HFpEF in the general population and there is a lack of evidence to support a specific drug regimen to treat HFpEF in the PD population.12,15 As explained above, evidence suggests that treating hypertension could be important for preventing HFpEF progression. Hypertension treatment involves recognition and control of volume overload, lowering dietary sodium intake, appropriate use of loop diuretics in the presence of substantial RRF, adaptation of dialysis regimen to peritoneal transporting status, and use of icodextrin solutions during long daytime dwell in APD or during the long overnight dwell in CAPD. Antihypertensive drug therapy should only be initiated when hypertension remains uncontrolled despite the adequate management of volume.
Although comparative efficacy and safety of different antihypertensive drug categories among patients on PD remains elusive, some clinical studies suggest using ACEIs/ARBs as first‑line agents.12 Further, RAAS blockers are associated with slower decline in RRF and also with peritoneal membrane function preservation.49
According to the ISPD guidelines, in patients with LVH or dilated cardiomyopathy, treatment with a beta‑blocker should be considered.12 In hemodialysis patients, its use improved left ventricular remodeling and functional class in patients with dilated cardiomyopathy sustained for a period of 24 months and showed significant benefit in cardiovascular mortality, and HF related and all‑cause hospitalizations.50 Studies evaluating the influence of beta‑blockers in LVH and diastolic disfunction in PD patients are lacking.
In patients already receiving ACEIs/ARBs treatment with MRAs should be considered. Spironolactone has been shown to significantly reduce the progression of left ventricular mass index over a 24‑month period in PD patients compared with controls. There was no significant difference in the occurrence of hyperkalemia between the two groups.51
As stated above, anemia is a risk factor for LVH and HF. There is insufficient data to recommend a different approach and Hb goals in patients with or without HF, so the recommendation for anemia treatment in PD patients should follow KDIGO 2012 recommendations.12,47 Iron deficiency is associated with a worse prognosis in HF patients. Intravenous iron supplementation has been studied in patients with HF and iron deficiency (serum ferritin <100 mg/L or ferritin between 100 and 299 mg/L and transferrin saturation <20%) both with and without anemia. In the FAIR‑HF trial, intravenous ferric carboxymaltose has been shown to improve self‑reported patient global assessment and NYHA class.52 Although these studies were not realized in PD patients, it seems reasonable to give special attention to iron metabolismo in patients with HF even without anemia.
The correction of abnormalities on chronic kidney disease - mineral bone disease (CKD‑MBD) metabolism might have beneficial effects on LVH. In patients with secondary hyperparathyroidism on hemodialysis, intravenous calcitriol induced regression in myocardial hypertrophy and improved cardiac systolic and diastolic function.53 Results with cinacalcet are contradictory. The EVOLVE trial, which included 2,602 patients with secondary hyperparathyroidism on hemodialysis, reported that treatment with cinacalcet seemed to have beneficial effects on cardiovascular end points in older patients.54 A posterior meta‑analysis reported that treatment with cinacalcet was not associated with a beneficial effect on cardiovascular outcomes, such as LVH, CHD or cardiac function.55 Supplementation with cholecalciferol could be considered.30
Restoration and maintenance of sinus rhythm is preferred when atrial fibrillation occurs in patients with HFpFE. When this cannot be achieved, rate control becomes essential, preferably with a cardio selective beta‐blocker. Correction of valvular disease should also be considered in appropriate patients.12,15
Novel therapies targeting inflammation, oxidative stress, and uremic toxins could be the next step for uremic cardiomyopathy treatment.6 A few studies have investigated the effect of antioxidants such as Vitamins C, E, statins, omega‐3 fatty acids, and N‐acetylcysteine.
Oral administration of N‑acetylcysteine was the most encouraging and was associated with suppression of oxidative stress, inhibition of local and systemic inflammation, prevention of loss of RRF and prevention of peritoneal membrane dysfunction.56,57
Treatment of heart failure with reduced ejection fraction
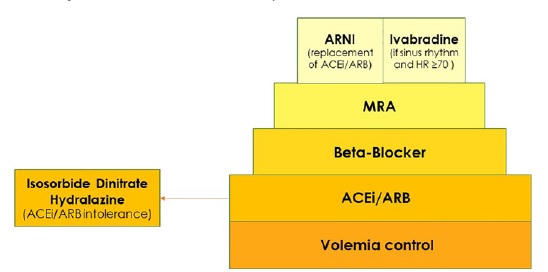
Treatment options for HFrEF are better established (Figure 1). According to ESC guidelines, ACEI should be considered in patients with stable CHD to prevent or delay the onset of HF. Hydralazine and isosorbide dinitrate may be considered in symptomatic patients with HFrEF who cannot tolerate neither an ACEI nor an ARB to reduce the risk of death. Beta‑blockers are recommended in patients with a history of myocardial infarction, to prevent or delay the onset of HF.15
ACEI - angiotensin-converting enzime inhibitor; ARB - angiotensin-receptor blocker; ARNI - angiotensin receptor neprilysin; HR - hearth rate; MRA - mineralcorticoid receptor antagonista
Concerning the treatment with MRA on PD patients with HFrEF, spironolactone improved left ventricular ejection fraction without a significant increase in potassium levels.51 According to ESC guidelines, ivabradine should be considered to reduce the risk of HF hospitalization or cardiovascular death in symptomatic patients with ejection fraction ≤35%, in sinus rhythm and a resting heart rate over or equal to 70 bpm despite treatment with na evidence‑based dose of beta‑blocker, ACEI/ARB and an MRA.15 At present, no data for patients with glomerular filtration rate inferior to 15 mL/min/1.73 m2 is available. Although efficacy and safety may be preserved in these patients too, more trials are needed, and ivabradine should be used carefully or avoided in ESKD patients.58
A new therapeutic class of agents acting on the RAAS and the neutral endopeptidase system has been developed, the angiotensin receptor neprilysin inhibitor (ARNI). By inhibiting neprilysin, the degradation of natriuretic peptides and bradykinin is slowed. Elevation of circulating A‑type natriuretic peptide (ANP) and NT‑proBNP is a compensatory mechanism that leads to vasodilation, natriuresis and diuresis, lowers blood pressure, lowers sympathetic tone, and reduces aldosterone levels. The natriuretic peptide system works antagonistically to the RAAS and has favorable effects on the pathogenesis of HF, enhancing myocardial relaxation and preventing remodeling.59 The association of sacubitril, an ARNI, and valsartan is recommended by the ESC guidelines as a replacement for na ACEI to further reduce the risk of hospitalization and death in patients with HFrEF who remain symptomatic despite optimal treatment with an ACEI, a beta‑blocker and an MRA.15 A case report on a hemodialysis patient showed that sacubitril/valsartan was safe and improved heart failure symptoms.60 There is lack of evidence regarding the use of this drug on PD patients and more studies are needed to assess the safety and efficacy of this promising drug class.
CONCLUSION
HF and CVD remain one of the main causes of morbidity and mortality in PD patients. The importance of simple interventions such as sodium and water restriction, exercise, smoking cessation, and lifestyle modification cannot be overemphasized. Since hypervolemia is the main risk factor for HF in PD patients, prescriptions need to be tailored to achieve euvolemia. Anemia, CKD‑MBD and inflammation status improvement should be considered as fundamental parts of HF treatment.
More studies are needed to assess the efficacy and safety of the new drugs available for HF treatment in PD patients.