INTRODUCTION
Novel coronavirus disease (COVID-19) is a newly discovered disorder declared a global pandemic by the World Health Organization (WHO) on March 11, 2020. Its pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is highly transmissible and primarily injures the vascular endothelium1. While it presents predominantly as an acute lung illness, patients eventually develop a multiorgan failure among which the kidney lesion can be particularly challenging2.
Clinical manifestations vary, and the exact mechanism of the lesion is still unclear. We describe a clinical case of acute kidney injury in a severe SARSCov 2 infection and report its´ histological findings.
CASE REPORT
We present the case of a 49-year-old man from India, admitted to our hospital with bilateral SARS-CoV-2 pneumonia in March/2020.
He had no previous medical conditions except for being slightly overweight (BMI 27 kg/m2). He presented at the Emergency Room with fever (39.5ºC), cough and myalgia for three days, pulmonar auscultation was unremarkable, and his mental status was normal.
On admission, blood pressure was 85/61 mmHg and heart rate was 85 bpm. His laboratory tests showed hemoglobin 11.4 g/dL, leukocyte count 3.04 x 109/L with lymphopenia 0.57 x 109/L, platelet count 138 x 109/L, serum creatinine (SCr) 0.83 mg/dL, urea 18 mg/dL, lactate dehydrogenase 429 U/L and C-reactive protein 9.97 mg/dL. Pulmonary X-ray evidenced a bilateral flocculated pneumonia, and the nasopharyngeal
swab was positive for SARS-CoV-2 by nucleic acid amplification teste (RT-PCR).
He started treatment with lopinavir/ritonavir, hydroxychloroquine, azithromycin, amoxicillin/clavulanic acid and ascorbic acid (6g/day for a total of 56g) upon admission. However, due to clinical deterioration, he required mechanical ventilation and was admitted to the ICU within 24 hours of admission. Blood cultures, Streptococcus pneumoniae and Legionella pneumoniae antigens and RT-PCR for other respiratory vírus were all negative.
His renal function declined rapidly as evidenced by an increase of SCr to 2.59 mg/dL and a decrease in urine output within 48 hours and peaking at SCr 5.52 mg/dL on day 5 with anuria. No diarrhea or other factors leading to hemodynamic instability were noted; direct nephrotoxic drugs were not given, and a maximum noradrenaline dosage of 6 mcg/min was administered. Intermittent renal replacement therapy was maintained. Lopinavir/ritonavir was stopped on day 6.
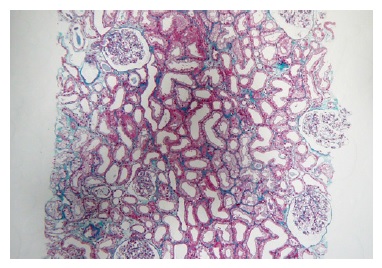
A kidney ultrasound was performed on day 7, with no lesions found and all complement, ANA, ANCA, anti-GBM and haptoglobin within normal range. Unfortunately, urinalysis was unavailable due to laboratory constraints - they refused urine samples analysis on COVID-infected patients at this time. A kidney biopsy was performed on day 11 to understand the cause of AKI. Paraffin-embedded tissue revealed 30 glomeruli and some medium-sized arteries.
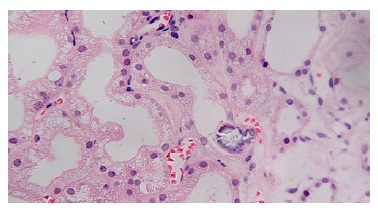
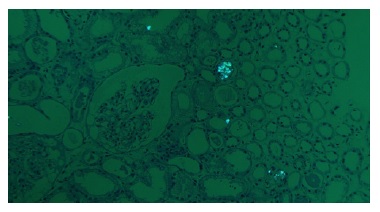
Extensive acute tubular necrosis and proximal tubular isometric vacuolization were notable (Figure 1 and Figure 2). Numerous tubules showed birefringent crystals under polarized light, which is a typical feature of calcium oxalate crystals (Figure 3 and Figure 4). Glomeruli and interstitium were normal. Immunofluorescence was negative. Electron microscopy was performed using paraffinembedded tissue. After paraffin removal and re-processing for transmission electron microscopy, thin sections of the glomeruli
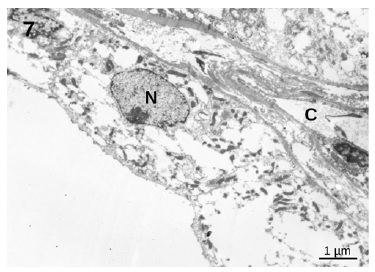
showed an essentially normal structure. In glomeruli, 100 nm virus-like particles could be observed in the capillary lumens (Figure 5). Observation of the proximal tubules revealed extensive vacuolization and degenerated epithelial cells with few particles with dimensions of approximately 100 nm that could represente virus particles (Figures 6 and 7). Some capillaries were clogged with a fibrillar coagulum containing dense masses of unknown nature (Figure 8).
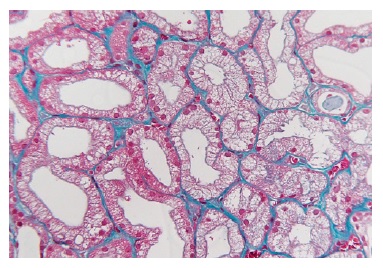
Figure 2 Masson’s trichrome x400. Acute tubular necrosis with multiple isometric vesicles in the tubular epithelium
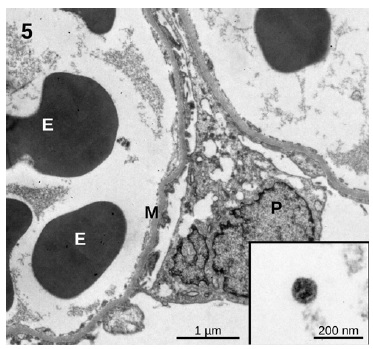
Figure 5 Glomerulus. The capillaries remain open and no significant changes in structure can be observed. A few circulating spherical particles with an approximate size of 100 nm are compatible with virus particle morphology
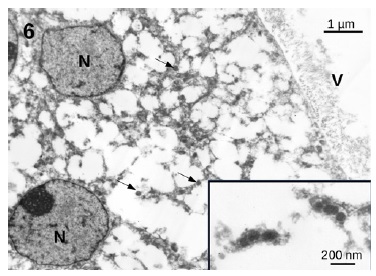
Figure 6 Proximal convoluted tubule. Notice the extensive vacuolization of the cells and the presence of small 100 nm particles (arrows) that could represente virus particles
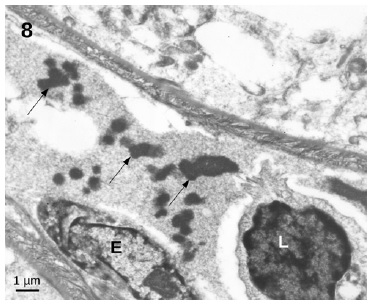
Figure 8 Capillary in the connective tissue filled by a fibrillar cloth containing dense inclusions of unknown nature (arrows)
Real-time PCR on paraffin embedded kidney tissue was negative for SARS-CoV-2 RNA. The patient was maintained on supportive therapy, ascorbic acid was discontinued and hemodialysis continued for a month. Ultimately the patient recovered and was discharged with serum creatinine 1.3mg/dL, and was lost to follow up.
DISCUSSION
Acute kidney injury (AKI) is defined as an increase in SCr by 0.3 mg/dL within 48 hours or a 50% increase from the baseline within 7 days, according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria.3 Thus, according to our patient values, AKI stage III was recognized.
The first COVID-19 worldwide reports suggested a low incidence of kidney damage, but a higher frequency was later documented and AKI was classified as an independent risk factor for these patients’ mortality.4 Most cases presented with microhematuria, mild proteinuria and increased blood urea nitrogen and serum creatinine with glomerular filtration rate decrease, all portending a worse prognosis.5
Kidney involvement is most likely multifactorial, with sepsis leading to cytokine storm, diffuse proximal tubular injury or direct celular injury by the virus via angiotensin-converting enzyme receptors being the predominant triggers6,7. Less commonly, iatrogenic damage by excessive diuretics and antiviral agents can occur.4 Notwithstanding the limited knowledge on the physiopathology and histology of the disease, six necropsy studies from China reported interstitial infiltration and collapsing glomerulopathy secondary to viral infection5. More recent biopsy studies showed multiple cases with glomerular involvement, particularly in patients with high risk APOL1 genotypes, from collapsing glomerulopathy to immune mediated conditions8. In all these conditions, cytokine-derived damage seems to be the culprit, since no direct viral infection was proven.
It is important to note the probable existence of a selection bias, whereby only patients with proteinuria and/or hematuria and kidney injury are biopsied. In most patients, the most probable injury mechanism is probably acute tubular necrosis due to sepsis, hypoxemia, clotting dysregulation and hemodynamic instability9,10.
Our case study, however, showed mainly tubular epithelium injury with acute tubular necrosis and multiple isometric vacuolization, possible due to direct viral infection, even without interstitial inflammation.
However, this vacuolization is a non-specific lesion that can be seen in other pathologic conditions. Capillary clogs were also apparent and may be the cause of ischemia and kidney damage; however glomerular ischemia was not apparent. The origin of these clogs is unfortunately not completely understood, and is perhaps associated with viral infection or sepsis. The ultrastructural evaluation of the tubular proximal epithelium shows that there were few virus-like particles. The observed particles are of the right size for coronavirus, although details such as spikes could not be identified in the damaged sample pre-processed for paraffin histology. This supports our hypothesis of direct renal infection, even with negative RT-PCR. Negative PCR may be due to a low viral load, and is consistent throughout the literature. It is also not clear if those inclusions are viral related or a result of cellular damage11.
However, existence of ACE2 receptors in both proximal tubule and the glomeruli make direct viral infection plausible but still inconclusive.
Because our patient was taking a high dose of Vitamin C, the presence of calcium oxalate crystals raises the hypothesis of vitamin C-induced oxalate nephropathy. Vitamin C is metabolized into oxalate and can induce AKI by precipitation of calcium oxalate in the renal tubules.
There are also a few reports of anecdotal kidney toxicity of ritonavir12. Among them, a patient with two AKI episodes after starting two different antiretroviral therapies also took ritonavir and his histological alterations mimicked our patient’s findings, such as tubular vacuolization13.
The absence of urinalysis hinders a possible clinical correlation with other findings. We present multiple different findings in this kidney specimen. Even if it was impossible to affirm the existence of direct viral infection due to negative PCR, virus-like particles were observed. Importantly, capillary clogs, which may represent indirect viral damage due to ischemia were noted. Calcium oxalate crystals and tubular vacuolization secondary to putative supportive therapy highlight the potential danger in unproven treatment strategies.