INTRODUCTION
Dysproteinemia occurs when B or plasma cell clones produce pathogenic monoclonal immunoglobulins and/or light chains.1 Multiple myeloma (MM), chronic lymphocytic leukemia and Waldenström macroglobulinemia (WM) can cause dysproteinemia-associated kidney diseases. Pathological monoclonal immunoglobulins can damage multiple organs. The kidney is one of the most affected organs because it is exposed to a large blood flow and consequently to a large concentration of immunoglobulins. Moreover, the unique renal pH and electrolyte concentrations can alter the characteristics of these proteins and contribute to their nephrotoxicity.2,3
MM is the most common hematologic malignancy, with an anual incidence of between 3.6-7.2 per 100 000 patients.4 Around 20%-30% patients with recently diagnosed MM will present an estimated glomerular filtrated rates below 30 mL/min/1.73 m2 and up to 5% will require dialysis at the time of diagnosis.5 In fact, kidney injury is one of the defining criteria for MM according to the International Myeloma Working Group.6 The most common renal injury associated with MM is light chain cast nephropathy that results from the accumulation of insoluble casts formed from the binding of monoclonal free light chains and Tamm-Horsfall proteins.4 The typical presentation of light cast nephropathy is acute kidney injury (AKI) or rapidly progressive renal failure.5,7 Amyloidosis, resulting from the glomerular deposition of fibrils of degraded immunoglobulins, is the most frequent glomerular lesion associated to MM,8 however, is not always associated with MM and can result from other diseases.7 Deposition of light chains can lead to monoclonal immunoglobulin deposition disease which frequently presents as AKI and nephrotic range proteinuria.5,7 Less frequent glomerular involvement of MM is membranoproliferative and immunotactoid glomerulonephritis.5,7 Tubular toxicity of light chains can cause global proximal tubular dysfunction, named Fanconi syndrome.5,7
WM is a lymphoplasmacytic lymphoma that secretes an IgMmonoclonal protein and kidney involvement is seen in about 3% of patients.9 Monoclonal gammopathy of renal significance (MGRS) is defined when monoclonal protein-related kidney lesion occurs in the absence of overt malignancy and treatment of dysproteinemia is indicated in these patients.5,7
The aim of this study was to describe and analyze the characteristics and outcome of the patients diagnosed with dysproteinemia-associated kidney disease with initial renal presentation.
MATERIAL AND METHODS
We retrospectively analyzed hospitalized patients admitted to the Department of Nephrology and Renal Transplantation at Centro Hospitalar Universitário Lisboa Norte, in Lisbon between 2015 and 2020, who were diagnosed with dysproteinemia-associated kidney disease. We included adult patients diagnosed with MM, amyloidosis and WM during hospitalization.
Patients with previously diagnosed dysproteinemia were excluded. MM was diagnosed according to MM diagnosis criteria according to International Myeloma Working Group as shown in Table 1.6 Amyloidosis was diagnosed by abdominal fat or kidney biopsy by congo-red stain. WM was diagnosed according to WM definition of Second International Workshop on Waldenström’s Macroglobulinemia.10 Data was collected from individual electronic clinical records. The following variables were analyzed: age, gender, comorbidity, laboratorial parameters, nephrological syndrome presentation, renal biopsy pattern and performed chemotherapy regimens.
Analyzed outcomes included need for renal replacement therapy, dialysis dependence at discharge, infections during hospitalization, one-year mortality and one-year dialysis dependency. Categorical variables were described as the total number and percentage for each category, whereas continuous variables were described as mean ± standard deviation.
Table 1 Multiple Myeloma Diagnosis Criteria according to International Myeloma Working Group
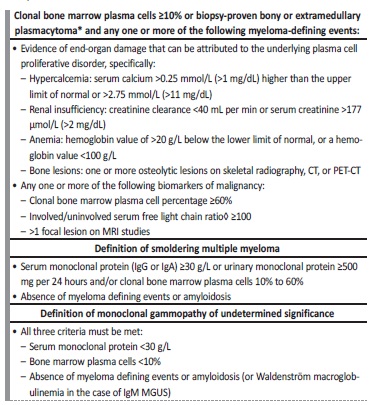
TC - computed tomography; PET-CT - positron emission tomography-computed tomography; MGUS - monoclonal gammopathy of undetermined significance; MRI - magnetic resonance imaging
Nephrotic syndrome (NS) was defined by the presence of heavy proteinuria (protein excretion greater than 3.5 g/24 hours), hypoalbuminemia (less than 3.5 g/dL), and peripheral edema. AKI was defined as na abrupt decrease in kidney function according KDIGO criteria: increase in serum creatinine by ≥0.3 mg/dL (≥26.5 micromol/L) within 48 hours or increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior seven days. Chronic kidney disease (CKD) was defined as the presence of kidney damage (urinary albumin excretion of ≥30 mg/day or equivalent) or decreased kidney function (eGFR <60 mL/min/1.73m2) for three or more months. The CKD stage classification used was KDIGO classification. Rapidly progressive renal failure (RPRF) was defined by a progressive kidney damage over weeks or months not meeting AKI criteria. Full recovery of renal function was defined by reaching 75% of previous baseline creatinine, partial recovery of renal function by reaching at least 25% of previous baseline creatinine and not recovery of renal function by dialysis dependence or not reaching 25% previous baseline creatinine.
RESULTS
The studied population included thirty patients with dysproteinemia-associated kidney disease. Patients’ demographic and clinical data are described in Table 2. Fifteen patients were male (50.0%). Mean age was 71.5 ± 12.44 years. Twenty-one patients had hypertension (70.0%), nine patients (30.0%) had diabetes mellitus, six patients (20.0%) had solid neoplasia and five patients (16.7%) had cardiac failure. Only four patients (13.3%) had previous monoclonal gammopathy of unknown insignificance.
Table 2 Demographic and clinical data of studied population
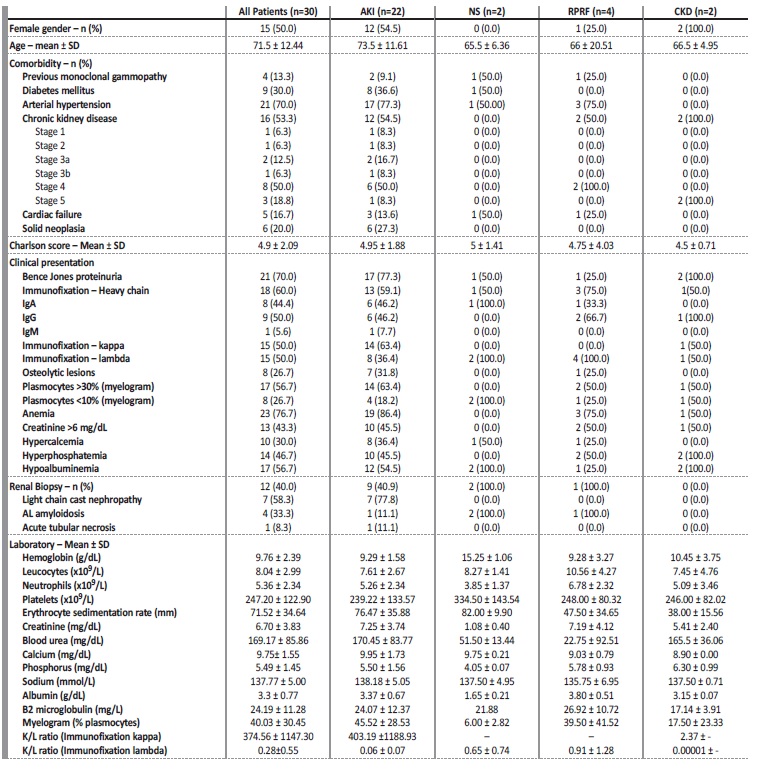
AKI - acute kidney injury; NS - nephrotic syndrome; RPRF - rapidly progressive renal failure; CKD - chronic kidney disease
These comorbidities reflected a high Charlson score: mean 4.9 ± 2.09. Majority of the patients had anemia (n=23, 76.7%). Hypercalcemia and hyperphosphatemia were also common, 30.0% (n=10) and 46.7% (n=14) of the patients, respectively. Thirteen patients (43.3%) presented with serum creatinine above 6 mg/dL. Bence jones proteinuria was present in twenty-one patients (70.0%). A positive monoclonal light chain immunoglobulin serum immunofixation was present in all patients with equal prevalence of kappa or lambda chains. Eighteen patients (60.0%) had a positive monoclonal heavy chain immunoglobulin serum immunofixation. The most common heavy chain immunoglobulin was IgG (9 patients, 50.0%), followed by IgA (8 patients, 44.4%). Only one patient (5.6%) had IgM, corresponding to the patient with WM diagnostic. Myelogram was performed in all patients. Seventeen patients (56.7%) presented with >30% of plasmocytes and eight patients (26.7%) with < 10% of plasmocytes. MM was diagnosed in twenty-six patients. Three patients were diagnosed with MGRS and one patient had WM.
The most common renal manifestation at presentation was AKI in 73.3% of patients (n=22). Rapidly progressive renal failure was presented in four patients (13.3%), CKD in two patients (6.7%) and NS in two patients (6.7%). Kidney biopsy was performed in 12 patients. The histological patterns were light chain cast nephropathy (n=7), followed by light chain amyloidosis (AL amyloidosis) (n=4) and acute tubular necrosis (n=1). All patients who presented with NS had AL amyloidosis on biopsy.
The description of treatment and follow-up are shown in Table 3. Twenty-nine patients (96.8%) were treated with chemotherapy and the most used drugs in chemotherapy regimens was dexamethasone, bortezomib, cyclophosphamide and lenalidomide. Twenty-two patients (73.33%) required renal replacement therapy (RRT). Six (27.3%) patients were treated with high cut-off dialyzers (all of them had kidney biopsy: five had light chain cast nephropathy and the other acute tubular necrosis): two patients had renal function recovery (full recovery n=1; partial recovery n=1) and four patients had no renal function recovery.
Table 3 Treatment performed and follow-up by nephrological syndrome
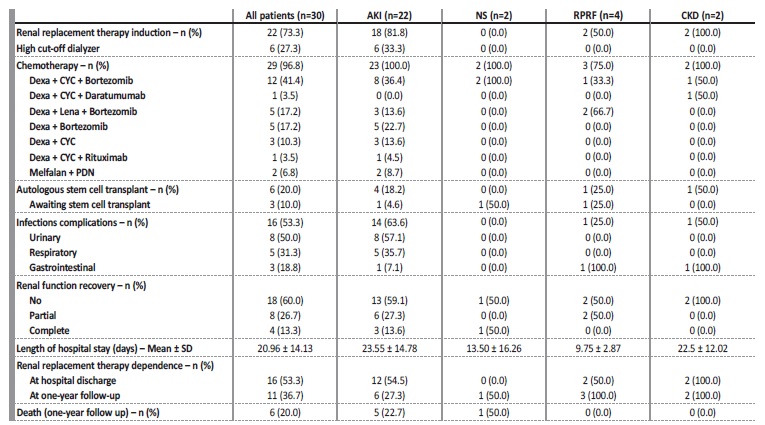
AKI - acute kidney injury; NS - nephrotic syndrome; RPGN - rapidly progressive renal failure; CKD - chronic kidney disease; Dexa - dexamethasone; CYC - cyclophosphamide; B - bortezomib; PDN - prednisolone; Lena - lenalidomide
Mean length of hospital stay was 20.96 ± 14.13 and infections during hospitalization occurred in sixteen patients, predominantly urinary and respiratory infections (50.0% and 31.3%, respectively). At hospital discharge, four patients (12.9%) had full recovery of renal function and sixteen (53.3%) remained RRT dependence. At one-year follow-up, six patients died (20.0%), eleven patients (36.7%) were in RRT. More precisely, eight patients (50.0%) of sixteen which were RRT dependence at hospital discharged remained RRT dependence, four (25.0%) were not in RRT and four (25.0%) have died; three patients (21.4%) of the fourteen which were not RRT dependence at hospital discharge were on RRT, two (14.3%) have died and 9 (64.3%) remained not RRT dependente (Table 4). The mean creatinine at one-year follow-up of the patients were not in RRT was 2.11 ± 1.32 mg/dL. Of the patients who died, according to myelogram: three had above 30% and one below 10% of plasmocytes, and according to light-chain and heavy chain immunoglobulin immunofixation: three were kappa, three were lambda and four had IgG. During follow-up, six patients had autologous stem cell transplant and three patients are still awaiting stem cell transplant. Table 5 shows the demographic, clinical data and treatment performed and outcomes according to dysproteinemia diseases.
Table 4 Influence of renal replacement therapy on prognostic at one-year follow-up
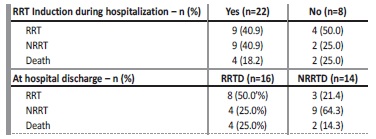
RRT - renal replacement therapy; NRRT - non renal replacement therapy
Table 5 Demographic, clinical data and treatment performed by dysproteinemia disease

MM - multiple myeloma; AL - AL amyloidosis; MGRS - gammopathy renal significance; WM - Waldenström macroglobulinemia; RPRF - rapidly progressive renal failure; Dexa - dexamethasone; CYC - cyclophosphamide; B - bortezomib; PDN - prednisolone
Acute kidney injury
Twenty-two patients presented with AKI. Of these, 54.5% were female. Age at presentation was 73.5 ± 11.61 years old. At presentation, mean hemoglobin was 9.29 ± 1.68 g/dL, and erythrocyte sedimentation rate was 76.47 ± 35.88 mm. Mean creatinine was 7.25 ± 3.74 mg/dL, 10 patients presenting with creatinine greater than 6 mg/dL, and mean urea was 170.45 ± 83.77 mg/dL. Calcium at presentation was 9.95 ± 1.73 mg/dL, and albumin 3.37 ± 0.67 g/dL. Mean β2 microglobulin was 24.07 ± 12.37 mg/L, kappa/lambda ratio was 403.19 ± 1188.93 in kappa dysproteinemia and was 0.06 ± 0.07 in lambda. Mean plasmocytes percentage in myelogram was 45.52 ± 28.53%. Most patients (n=13) had a monoclonal heavy chain determined by immunofixation. Kappa light chain was identified in 63.4% (n=14) of patients. Fourteen patients (63.4%) had over 30% plasmocytes on myelogram. Most patients (81.8%) required renal replacement therapy, and high cut-off dialyzers were used in six (33.3%) of these patients.
All patients underwent chemotherapy, and the most frequently used combination was dexamethasone with cyclophosphamide and bortezomib in eight patients. Five patients were treated with dexamethasone and bortezomib and three patients with dexamethasone, lenalidomide and bortezomib. The remaining patients received other combinations of chemotherapy. Four patients (18.2%) were submitted to autologous stem cell transplantation. Thirteen patients (59.1%) did not recover renal function. Twelve patients remained dependent on RRT at hospital discharge, four of them remain RRT-dependence in one-year follow-up, four were non RRT-dependence and four had died. Ten patients were not RRT-dependent at hospital discharge, but two patients started RRT and two had died in one-year follow-up.
Rapidly progressive renal failure
Four patients presenting with rapidly progressive renal failure were included. Most were male (75.0%). Age at presentation was 66 ± 20.51 years old. One patient had previous monoclonal gammopathy. Half of the patients (n=2) already had CKD at presentation, both of them on stage 4. At admission, mean hemoglobin was 9.28 ± 3.27 g/dL, erythrocyte sedimentation rate was 47.50 ± 34.65 mm, creatinine was 7.19 ± 4.12 mg/dL, and urea was 222.75 ± 92.51 mg/dL. When it comes to electrolytes calcium was 9.03 ± 0.79 mg/dL and phosphorus 5.78 ± 0.93 mg/dL. Myelogram revealed a 39.50 ± 41.52 % of medullary plasmocytes.
All patients with rapidly progressive renal failure had a lambda light-chain monoclonal immunoglobin positive immunofixation and kappa/lambda ratio was 0.91 ± 1.28. The mean β2 microglobulin was 26.92 ± 10.25 mg/L. At presentation, three patients (75.0%) had a heavy chain identified on immunofixation. The most common was IgG in two patients (66.7%). Three patients (75.0%) started chemotherapy.
One patient was submitted to autologous stem cell transplantation. Two patients (50.0%) required renal replacement therapy and did not recover renal function. At a one-year follow-up, 3 patients were dependente of renal replacement therapy. No patients died during follow-up.
Chronic kidney disease
Two patients presented with CKD stage 5 at presentation. Both were female. Mean age at presentation was 66.5 ± 4.95 years old. None had previous monoclonal gammopathy. Both patients presented with Bence Jones proteinuria, hyperphosphatemia (6.30 ± 0.99 mg/dL) and hypoalbuminemia (3.15 ± 0.07 g/dL), but neither had hypercalcemia or osteolytic lesions at presentation. One patient had IgG lambda type in immunofixation, and the other patient had kappa light chains without heavy chain. One patient had more than 30% plasmocytes on myelogram while the other had less than 10%. Mean creatinine was 5.41 ± 2.40 mg/dL and mean urea was 165.5 ± 36.06. β2 microglobulin was 17.14 ± 3.91 mg/L. One patient had a positive kappa light-chain monoclonal immunoglobulin immunofixation with kappa/ lambda ratio 2.37 and the other had a lambda light-chain positive immunofixation with kappa/lambda ratio 0.00001. At presentation, erythrocyte sedimentation rate was 38.00 ± 15.56 mm. Both patients required renal replacement therapy and did not recover. Chemotherapy was started in both patients. None of these patients died.
Nephrotic syndrome
Two patients presented with NS. Both had AL amyloidosis finding in kidney biopsy and had lambda light-chain monoclonal immunoglobulin positive immunofixation with a kappa/lambda ratio 0.65 ± 0.74. One patient had an IgA heavy chain identified on immunofixation. Both patients had less than 10% plasmocytes on myelogram, with a mean 6.00 ± 2.83 % of plasmocytes. Mean age was 65.5 ± 6.36 years old and both patients were male. One had a previous diagnosis of monoclonal gammopathy. At presentation, mean creatinine was 1.08 ± 0.40 mg/dL, mean urea was 51.50 ± 13.44 mg/dL. Erythrocyte sedimentation rate at admission was 82.00 ± 9.90 mm. No patient had osteolytic lesions, hypercalcemia or anemia. Chemotherapy was started in both patients with dexamethasone, cyclophosphamide plus bortezomib or daratumumab.
No patients required renal replacement therapy during admission, although one patient presented renal function deterioration and started dialysis during follow-up, and ultimately died within one year.
DISCUSSION
The exclusion of dysproteinemia is part of the assessment of AKI and CKD because clone-directed therapy will induce clinical improvement of renal disease in most patients.11,12 It is also important to be aware of all possible renal manifestations because each of them may require individual treatment. For example, patients with amyloidosis usually present with NS which requires diuretic management, which is potentially contraindicated in light chain cast nephropathy. We decided to include dysproteinemia-associated kidney diseases non-MM that also affect the kidney, such as WM and MGRS (patient with dysproteinemia disease which do not meet MM diagnosis criteria or other hematologic disease).
This particularity of our study differs from other similar studies published and highlights the need to investigate all dysproteinemias. Over five years, we identified thirty patients who presented with kidney involvement, admitted to the Department of Nephrology and Renal Transplantation at Centro Hospitalar Universitário Lisboa Norte and were diagnosed with dysproteinemia.
Dysproteinemia diseases are more prevalent in older patients.5 The mean age of MM diagnosis is 64 to 74 years, only 10 and 2 percent of patients are younger than 50 and 40 years, respectively.13,14,15This is similar to the mean age population in our study. Most patients with MM present with constitutional symptoms (fatigue, weight loss) and skeletal pain (especially back pain) and renal impairment usually affects up to 50% of patients.13 MM often presents with hypercalcemia, cytopenia (mainly anemia, less frequently leucopenia and thrombocytopenia), osteolytic lesions and low-grade proteinuria (usually <3 g/L).13
In our study, anemia was the most common laboratorial finding in MM and osteolytic lesion is only reported in MM group patients.
Renal disease in MM most often presents as renal insufficiency and proteinuria. About one half of patients with newly diagnosed MM have evidence of impaired renal function with a serum creatinine >1.3 mg/dL, and 15%-20% presents severe renal insufficiency with serum creatinine >2.0 to 2.5 mg/dL.13 As dysproteinemia diseases are more prevalente in older ages, dysproteinemia should be part of etiological investigation of AKI or NS without evident cause, even when absence of other symptoms in adult patients, mainly if older than 50 years old. Light chain cast nephropathy is the most common cause of kidney injury in MM and occurs when monoclonal free light chains bind and precipitate with Tamm-Horsfall protein in distal nephron.5,17 Tubular cast leads to a tubular occlusion and an intense immune response with giant cells reaction around the cast and interstitial inflammation.5,17,18AKI is the most common presentation in light chain cast nephropathy and in most cases occurs in patients with serum light chains level >100 mg/dL.17 In our cohort, all patients with light chain cast nephropathy present with AKI. Light chain cast nephropathy can also coexist with other kidney lesions, such as monoclonal immunoglobulin deposition disease (MIDD) and AL amyloidosis.18 The kidney prognosis of light chain nephropathy depends on significant reduction of free light chain.19,20Based on this, the EULITE and MYRE trials analyzed the impact of reduction of free light chains using high cut-off dialyzer on renal prognosis and their results will be discussed later.
Amyloidosis is the most common glomerular lesion associated with monoclonal gammopathy and MM, and usually presents as nephrotic syndrome and less frequently as CKD, AKI or RPRF.18,21,22In our cohort, half of the patients (n=2) with AL amyloidosis on kidney biopsy presents with nephrotic syndrome, one patient presents with AKI and other patient presents with RPRF. Therefore, because not always kidney amyloidosis manifest as nephrotic syndrome, it is important to be aware of other systemic manifestation such congestive heart failure, orthostatic hypotension, peripheral neuropathy, diarrhea, macroglossia, and bleeding diathesis, that can suggest amyloidosis.14,21,23Cardiac involvement is associated with worse prognosis and is less common when heavy chain is involved.17,21,23Lambda AL amyloidosis and renal insufficiency are also associated with worse prognosis.17,21About 10% of AL amyloidosis patients meet criteria for MM diagnosis basis on CRAB features, and in our cohort we identified one patient (25%) with MM plus AL amyloidosis which presents as AKI, however this patient do not have light chain cast nephropathy in kidney biopsy.24
Approximately one-third of patients with AL amyloidosis progress to CKD needing to start RRT and these patients had higher mortality in comparison of other causes of end-stage renal failure.17,25 Half of the patients with AL amyloidosis (n=2) started RRT after hospital discharged and both died in one-year follow-up, which corroborates the amyloidosis poor prognosis.
Other kidney lesions associated to dysproteinemia disease such as such MIDD, membranoproliferative glomerulonephritis, light chain proximal tubulopathy (that can manifest as Fanconi syndrome) and immunoctadoid glomerulonephritis are less common and were not reported in our cohort.5
The number of biopsies performed in our study is low because in most cases the decision to start chemotherapy was not dependente on biopsy findings and some patients had contraindications to biopsy, namely small kidneys. In our study the most common finding in kidney biopsy was light chain cast nephropathy, and according to previous studies this finding is the most prevalent and considered the major cause of renal failure in MM.5,16,26,27 Other mechanisms frequently involved in renal failure in MM patients are hypercalcemia, dehydration, recurrent tract urinary infection, nephrotoxic medication and intravenous contrast administration, and it is important to exclude such factors as a cause worsening renal function.28
In our study most patients with MM presented with AKI or rapidly progressive kidney failure, together corresponding to 86.66% and this can explain the mean creatinine level (6.70 mg/dL). We only observed IgG and IgA positive heavy chain immunoglobulin in MM group (seven IgG and seven IgA) which is in accordance with current knowledge (IgM, IgD and IgE being rare).13 The unique positive IgM heavy chain immunoglobulin immunofixation was observed in a patient with WM diagnosis, which is, by definition, an IgM dysproteinemia disease.
Eleven patients with MM diagnosis (42.3%) presented as free light chain only myeloma which is a higher prevalence than reported in literature.13 A possible explanation for this is the fact that all patients included in the study had kidney abnormalities, and free-light chain secreting MM are known to be highly associated with severe kidney injury.29 According to literature kappa light chain secretion is slightly more prevalent in MM and lambda light chain secretion is more prevalente in amyloidosis.5,13 Amyloidosis heavy chain secretion is less common and only count about 5% of the cases.5 Our data is according to this, as we observed a slightly higher prevalence of kappa light chain secretion in MM diagnosis and all patients with AL amyloidosis finding in kidney biopsy had lambda light chain secretion, with exception of the patient with MM plus amyloidosis diagnosis which secreted kappa light chain.
About 5% of all patients with newly MM diagnosed develop severe irreversible renal failure requiring dialysis support.5 In our study this percentage is much higher due to a bias selection, as we only selected patients with renal involvement at presentation. This might also explain the high number of dialysis dependence at one-year follow-up. In addition, despite aggressive treatment, progression to CKD with RRT dependence occurs up to 65% of patients with cast nephropathy within 3 months of diagnosis.18 Like amyloidosis, the MM patients on RRT had higher mortality in comparison with other causes of CKD.17,30
There are no difference between hemodialysis and peritoneal dialysis in terms of treatment-related complications.17,31,32
According to EULITE and MYRE trials, the use of high cut-off dialyzers showed conflicting results.33,34 The MYRE trial is a phase III multicentre controlled national clinical trial which analyzed, in 94 patients with myeloma cast nephropathy requiring dialysis, the efficacy of high cut-off dialyzers added to chemotherapy with dexamethasone, cyclophosphamide plus bortezomib (CVD). This trial show high dialysis independence at 6 and 12 months in high cut-off dialyzers group but it is not statistically significant at 3 months. The EULITE trial is a phase II multicenter randomized clinical trial which analyzed the efficacy high cut-off dialyzers in myeloma cast nephropathy associated to dexamethasone, doxorubicin and bortezomib chemotherapy and this trial include 90 patients with myeloma cast nephropathy dialysis dependent. In EULITE trial the high cut-off dialyzes group had no improvement in dialysis independence at 3 months. Of six patients treated with high cut-off dialyzers in our cohort, only one had full renal function recovery and this patient had acute tubular necrosis in kidney biopsy which could explain renal function recovery by itself, and considering the results of the recent clinical trials, high cut-off dialyzers are no longer used in our department.
Recovery of renal function is dependent on starting chemotherapy; therefore, it must be started as soon as possible.11,12In our study, patients started chemotherapy regimens immediately after the diagnosis of MM. Despite this, most of our patients had no renal recover and only a small number of patients presented complete renal recovery.
This might be explained by the prevalence of previous CKD and a high prevalence of dialysis need at presentation which is associated to worse outcomes.35,36Focusing on AKI and dialysis needs, Haynes et al. studied the clinical outcomes of 107 MM patients with AKI. They found three main protective factors: use of chemotherapy, sérum albumin >3.5 g/dL and dialysis independence (even in the patients which required dialysis at presentation).37
According to literature the introduction of combined chemotherapy regimens improves overall survival rate and the chemotherapy regimens in MM includes induction triple-drug chemotherapy regímen VRD (bortezomib, lenalidomide, dexamethasone) or DRD (daratumumab, lenalidomide, dexamethasone).28,38-42 VRD continues to be the first line but DRD is a reasonable alternative for patients with standard-risk MM who are not transplant candidates, especially for patients who cannot receive or tolerate bortezomib.42 VCD (bortezomib, cyclophosphamide, dexamethasone) is an option in light chain cast nephropathy with AKI because lenalidomide is typically avoided in AKI, unless the patient is refractory to other options.43 Two-drug chemotherapy regimen based in lenalidomide and dexamethasone is an option for frailty patients not eligible for three-drugs regimens.44
Due to the high prevalence of AKI or CKD, the chemotherapy regímen with cyclophosphamide was most used instead of lenalidomide regimens. Due to low performance status, eleven patients only received two-drug regimens chemotherapy and one patient was not treated with chemotherapy.
Autologous stem cell transplantation significantly extends survival and is now the treatment of choice for eligible patients.45 Patients on dialysis are suitable for autologous stem cell transplant, although increased morbidity and mortality is recognized. Autologous stem cell transplant is associated with renal recovery and freedom from dialysis.46-48Eligible criteria for autologous stem cell transplant in MM take into account age (patients above 70 years-old are illegible for autologous stem cell transplant) and performance status. Only six (20.00%) patients were submitted to autologous stem cell transplant, which is easily explained by the mean age (71.5 years) and Charlson score (4.9). Three patients were awaiting autologous stem cell transplant on the last follow up.
It is important to note the high prevalence of infectious complications during hospitalization (53.33% n=16), mostly in AKI group (14/16 87.5%). The immunosuppressive effect of chemotherapy performed could explain the number of infectious complications as well as the length of hospitalization. In the patients with infectious complications the mean days until hospital discharge was 25.81 ± 15.14 whereas in patients without infections complications was 15.43 ± 10.41. It is important to note that five of six patients submitted high cut-off dialyzers had infectious complications and, in this subgroup had more days of hospitalization (mean 30 days ± 8.43) in comparison to patients not submitted to high cut-off dialyzers (mean 18.71 days ± 14.48).
Six patients (20%) died within one-year. The mortality rate could result from the lack of chemotherapy with triple-drug regimens, and due to the high comorbidity rate and frailty of our patients. Dialysis dependence at discharge also contributes to worse prognosis.35-37
We recognize important limitations of our study. Firstly, the single center and retrospective nature of our study with a small sample limits the generalization of the results and secondly, we do not analyze death’s causes which made it impossible for us to draw more precise conclusions about the mortality associated with dysproteinemia. Nevertheless, our study demonstrates the heterogeneity of kidney involvement as the initial presentation of dysproteinemia disease, hence the need of high suspicion in the initial evaluation in patients with kidney abnormalities such as AKI, CKD, rapidly progressive renal failure or NS and high mortality associated to dysproteinemia kidney disease. In the future it maybe be important to screen dysproteinemia on routine analysis particularly in older patients, in order to have na earlier diagnosis and avoid progression of kidney damage and associated worse outcomes.
CONCLUSION
Dysproteinemia diseases can initially present with renal manifestation. Renal replacement therapy requirement and dependence at discharge appear to be related to higher mortality in these patients. In the future maybe it will be important to screen dysproteinemia on routine analysis, particularly in older patients without kidney damage.
The initiation of chemotherapy should be started as soon as possible in order to control the underlying disease and to improve overall outcomes.















