INTRODUCTION
Chronic kidney disease (CKD)-mineral and bone disorder (MBD) is a major complication in hemodialysis (HD) patients. One of its main characteristics is the abnormal metabolism of intact parathyroid hormone (iPTH). PTH is an 84 amino acid protein, which regulates mineral metabolism by targeting bone, kidney, and intestine, among other organs. The Kidney Disease Improving Global Outcomes (KDIGO) published in 2017 suggested maintaining serum iPTH levels in the range of two to nine times the upper normal limit for the assay in HD patients.1 Since the guideline was implemented, there has been na increased awareness of CKD-MBD, however, the level of evidence for this iPTH level recommendation is only C, which indicates the absence of randomized controlled trials (RCTs) showing that treatments to achieve a specific PTH level result in improved outcomes, including bone health, and the lack of specificity of PTH within the range of 150-300 pg/mL regarding bone histology and association with fractures.
Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) suggested that serum iPTH concentration ≥600 pg/mL is associated with higher all-cause mortality, but that PTH <100 pg/mL is associated with greater cardiovascular mortality in time-dependent models.2 Other studies have shown contradictory results regarding low PTH levels and prognosis in this group of patients.3-5To clarify optimal serum iPTH levels for a lower risk of mortality, we examined the association between diferente serum iPTH levels and all-cause and cardiovascular mortality in incident HD patients.
METHODS
We conducted a single-center retrospective cohort study of incidente HD patients at Centro Hospitalar do Médio Tejo, EPE HD unit from January 2013 to January 2020. Patients with previous parathyroidectomy, chronic inflammatory disease, active malignancy, who recovered renal function, lost follow-up, or switched to another renal replacement therapy were excluded. Patients were categorized according to serum iPTH levels, measured one month after HD initiation, in the mid-week HD session, in 4 groups: <150 pg/mL, 150-300 pg/mL, 301-600 pg/mL and >600 pg/mL. Clinical parameters were compared among groups, including age, sex, etiology of chronic kidney disease (CKD), comorbidities, vascular access type and medications with outcome influences. Other laboratory parameters measured during the same period, included phosphorus (P+), calcium (Ca2+), vitamin D (25-hydroxyvitamin D), bone-specific alkaline phosphatase (BAP), hemoglobin (Hgb), ferritin, albumin, C-reactive protein (CRP) and serum bicarbonate. iPTH was measured using the chemiluminescent microparticle immunoassay (CMIA), and albumin, P+ and Ca2+ by colometric assays.
The primary outcome was all-cause mortality and secondary outcome cardiovascular mortality, defined as death from cardiovascular disease including myocardial infarction, congestive heart failure and stroke.
Statistical analysis was performed using SPSS version 23.0 for Mac OS X. Continuous variables were presented as mean and standard deviation and nominal variables were presented as number (frequency) and percentage. A p value < 0.05 was considered statistically significant. Baseline characteristics and laboratory data were compared among groups using the ANOVA test for continuous variables and Chi-square test for categorical variables.
The association between iPTH levels at baseline and all-cause and cardiovascular mortality risk were examined using standard survival analysis methods. Survival time was calculated from the date of dialysis initiation until the date of death or end of follow-up (31 January 2022). Kaplan-Meier curves were developed for each group and compared by log-rank tests. Univariate and multivariate hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and cardiovascular mortality were estimated using the Cox proportional hazards risk model.
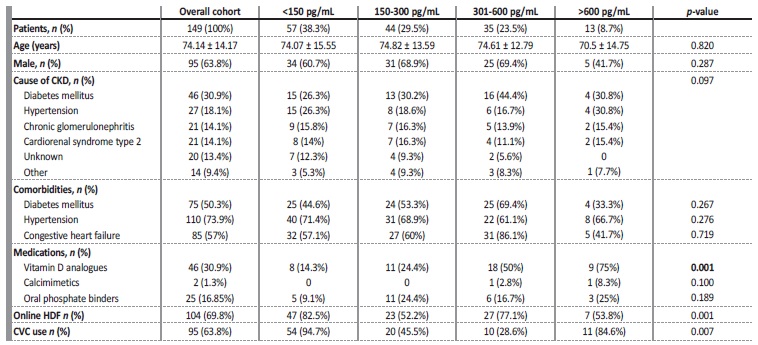
One hundred and forty-nine incident HD patients were recruited: 95 (63.8%) were male, 85 (57%) had congestive heart failure (CHF), 75 (50.3%) type 2 diabetes mellitus and 110 (73.9%) hypertension. Mean age was 74.14±14.17 years. The etiology of CKD was diabetic nephropathy in 46 patients (30.9%), hypertension in 27 (18.1%), cardiorenal syndrome type 2 in 21 (14.1%), chronic glomerulonephritis in 21 (14.1%), other in 20 (13.4%) and unknown in 14 patients (9.4%).
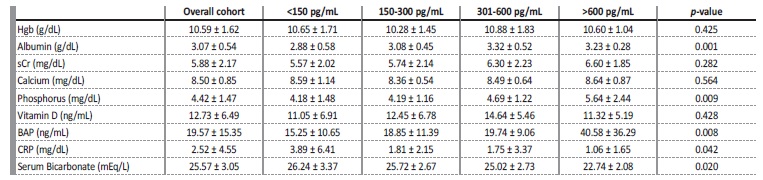
Forty-six (30.9%) patients were using vitamin D analogues, 25 (16.8%) oral phosphate binders and two (1.3%) calcimimetics. Most patients (n=104, 69.8%) were on online hemodiafiltration and had a central venous catheter (CVC) (n=95, 63.8%), one month after HD initiation. Fifty-seven (38.3%) patients had iPTH <150 pg/mL, 44 (29.5%) 150-300 pg/mL, 35 (23.5%) 301-600 pg/mL and 13 (8.7%)>600 pg/mL. No statistically significant differences were found in age, gender, comorbidities, etiology of CKD or HD modality among groups. A higher proportion of patients with PTH >600 pg/mL were treated with vitamin D analogues (Table 1). Patients with iPTH<150 pg/mL had higher sérum bicarbonate (p=0.020) and c-reactive protein (CRP) (p=0.042), lower albumin (p=0.001), serum phosphate (p=0.009) and bone-specific alkaline phosphatase (BAP) (p=0.008). No differences were found in hemoglobin (Hgb), serum creatinine (sCr), serum calcium (Ca2+) or vitamin D (Table 2).
Table 1 Baseline patient characteristics according to PTH level
p-values represent an ANOVA test for continuous variables and Chi-square test for categorical variables.
CKD, chronic kidney disease; HDF, hemodiafiltration; CVC, central venous catheter.
Table 2 Baseline patient analytical data according to PTH level
p-values represent an ANOVA test.
Hgb, hemoglobin; sCr, serum creatinine; BAP, bone-specific alkaline phosphatase; CRP, c-reactive protein.
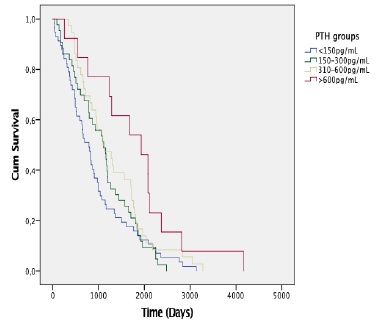
Among the patients enrolled in the study, there were 82 deaths over a follow-up period of 447 person-years. The mean follow-up was 3 years. The overall mortality rates by iPTH level at baseline were 22.2, 17.8, 16.6 and 7.7 per 100 person-years for iPTH <150 pg/mL, 150-300 pg/mL, 301-600 pg/mL and >600 pg/mL, respectively. Fig. 1 shows Kaplan-Meier curves for all-cause death according to groups stratified by iPTH levels. Patients with iPTH <150 pg/mL showed a higher incidence rate of all-cause death compared with those with iPTH 150-300 pg/mL, 310-600 pg/mL and > 600 pg/mL (log-rank test, p=0.022). Mean and median survival times confirmed a significant difference in disadvantage of PTH <150 pg/mL group (p=0.022), signifying that the observed difference in the survival curves was not due to chance (Table 3).
Table 3 Confidence intervals mean and median for survival time according to PTH level (all-cause mortality)
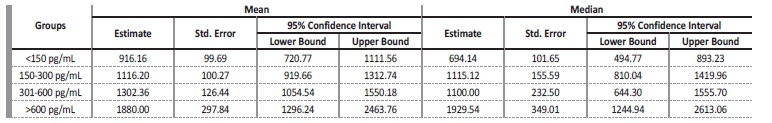
Table 4 Confidence intervals mean and median for survival time according to PTH level (cardiovascular mortality)
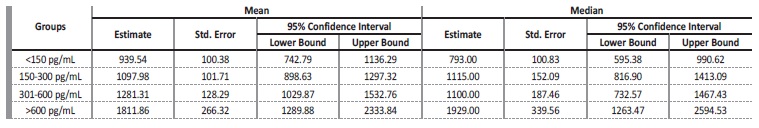
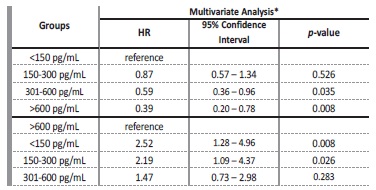
Multivariate Cox Regression showed that patients with iPTH<150 pg/mL had an increased risk of all-cause mortality when compared to those with iPTH 301-600 pg/mL (HR: 0.59; 95% CI: 0.36-0.96; p=0.035) and iPTH>600 pg/mL (HR: 0.39; 95% CI: 0.20-0.78; p=0.008) in both unadjusted and adjusted models for age, albumin, diabetes, CHF, and hypertension. No significant difference was found between iPTH<150 pg/mL and iPTH 150-300 pg/mL groups. When the reference was changed to iPTH>600 pg/mL, patients in this group had a decreased mortality risk when compared to those with iPTH<150 pg/mL (HR: 2.52; 95% CI: 1.28-4.96; p=0.008) and iPTH 150-300 pg/mL (HR: 2.19; 95% CI: 1.09-4.37; p=0.026), but not iPTH 300-600 pg/mL (Table 5).
DISCUSSION
The association between iPTH and mortality has been investigated as a biomarker of CKD-MBD for decades. Most studies reported na increased all-cause mortality in HD patients with higher serum iPTH levels.6,7 In addition to deleterious bone effects, secondary hyperparathyroidism has been linked to neurotoxicity, cardiomyopathy, impaired
insulin secretion, abnormal lipid metabolism, impaired immune function, metabolic acidosis, and impaired protein catabolism and anabolismo in HD patients.8,9
In recent years, however, some studies have shown that low iPTH levels are also associated with worse prognosis in HD patients, suggesting a U-shaped relationship between iPTH and mortality. Rhee et al reported low iPTH levels (<60 pg/mL) as independent risk factor for both aortic arch calcification and mortality in HD patients, but only patients with iPTH <60 pg/mL and 150-300 pg/mL were compared.10 Jean et al also suggested that low iPTH levels (<50 pg/mL) were associated with a significantly higher risk of mortality.11 Few studies, however, have compared mortality risk between patients with very low (<150 pg/mL) and very high (>600 pg/mL) iPTH levels. In our study, incident HD patients with iPTH <150 pg/mL had an increased risk of all-cause and cardiovascular mortality, when compared to those with iPTH>300 pg/mL, including those with iPTH >600 pg/mL, who presented the greatest difference in mortality risk, even after adjusting for potential confounding factors as age, albumin, and comorbidities (diabetes, CHF, and hypertension). When the reference group was changed to iPTH>600 pg/mL the difference remained for those with iPTH <300 pg/mL. Our results were comparable to those previously described by Yu et al who reported a higher incidence of mortality and non-fatal cardiovascular events in patients with low iPTH levels (<60 pg/mL), when compared to those with high iPTH levels (>600 pg/mL), irrespective of whether the patients were age-matched.12 In fact, patients with low iPTH levels have low bone turnover and formation, and a reduction in the buffering of circulating calcium and phosphorus, which predisposes toward arterial vascular calcification, increasing the risk of cardiovascular events and mortality.13,14
Furthermore, low iPTH levels have been associated with malnutrition and inflammation in HD patients, which may, in part, explain the higher mortality in these patients.15,16,17,18In our cohort, patients with iPTH < 150 pg/mL had indeed lower albumin and higher CRP levels, known markers of protein-energy wasting and inflammation in HD patients. Our results corroborate those of Roldão et al, who described lower albumin, body mass index, normalized protein catabolism rate and total cholesterol, and higher levels of CRP and interleukin-6 (IL-6) in a cohort of prevalent HD patients with PTH<150 pg/mL, when compared to those with PTH≥150 pg/mL.18 Protein-energy malnutrition is a common phenomenon in CKD patients, with an estimate prevalence of 18% to 75%, depending on dialysis modality, nutrional assessment tools and patient population,19 resulting from inadequate nutrition, anorexia, hypercatabolism and increased nutrient loss during dialysis. Most of these patients had also cachexia and increased levels of inflammatory cytokines, due to many underlying factors, including the uremic milieu, volume overload, decreased clearance of circulating proinflammatory cytokines, oxidative stress, and decreased levels of antioxidants, contributing to a chronic inflammation state and perpetuating hypercatabolism, muscle and fat wasting and endotelial damage with atherosclerosis, and increased cardiovascular risk and mortality.20 In our study, low iPTH levels were associated with malnutrion- inflamation-caquexia syndrome (MICS) in incident HD patients.
In fact, low PTH appears to decrease accumulation of adipose tissue, which may result in protein-energy malnutrition states. Additionally, in vitro secretion of PTH has been shown to suppress pro-inflammatory cytokines associated with poor outcomes in CKD patients,21 which may contribute to the increased cardiovascular and all-cause mortality risk in those with low PTH (<150 pg/mL).
In summary, iPTH levels were associated with all-cause and cardiovascular mortality in our cohort of incident HD patients. Our results suggest that patients with low iPTH at HD initiation had an increased mortality risk, which may reflect a frail group of patients with anorexia, sarcopenia, malnutrition, and inflammation.
There were some limitations in this study. First, this was a retrospective study with a small sample size. Second, iPTH levels were measured in a single time-point. Third, we do not evaluate additional clinical and analytical parameters related to MBD such as vascular calcification or fractures. Although we corrected for potential confounding factors in multivariate Cox regression analysis, further studies should be performed to confirm our findings.