INTRODUCTION
Central venous catheterization (CVC) is still frequently used for initiating hemodialysis in patients reaching end-stage kidney disease (ESKD)1-3 as it allows an almost immediate access for urgent start of hemodialysis. Although its use should be mostly reserved for situations in which kidney failure happens unpredictably or when attempts to
construct a vascular access have been unsuccessful,4 in many cases patients which are known to be in advanced stages of chronic kidney disease end up having to start hemodialysis through a CVC, which increases the risk of adverse outcomes.5 Together with prior cardiac rhythm device insertions,6,7prior CVC placement is therefore one of the main risk factors for occurrence of central vein stenosis (CVS) in the hemodialysis population.8
The role of CVC on the pathophysiology of CVS is still incompletely understood, but elements of vascular trauma, inflammation, coagulation, and remodeling are thought to be involved.9 Endothelium injury is thought to occur first upon CVC insertion and to be perpetuated by the continuous presence of an indwelling foreign body in the vascular lumen, generating turbulent blood flow and aggravating Wall vessel irritation and inflammation. Subsequent activation of leucocytes, production of myeloperoxidase and activation of the coagulation cascade may lead to smooth muscle cell proliferation and remodeling of the vein wall.10-12 Most frequently affected sites where CVS occurs include the subclavian and brachiocephalic veins and the superior vena cava.9 Though any CVC procedure bears the risk of CVS occurrence, studies suggest different CVC locations associate with diferente risk of stenosis, with subclavian CVCs being more prone to generating CVS than internal jugular ones.13 Increased number of CVC placements, as well as longer permanence of the CVC are also associated higher CVS prevalence.14,15
Presenting symptoms of CVS vary according to the site of stenosis.16 Arm edema and collateral venous circulation are commonly found in patients with CVS; in hemodialysis patients with an AV access, findings of AV access dysfunction may ensue, including an engorged, pulsatile fistula, positive arm elevation test, an increased difficulty in AV access puncture and an increased bleeding time due to elevated venous pressure as well as poor dialytic adequacy.9
In this work we aimed to evaluate previous central venous manipulation, clinical presentation, and complications in hemodialysis patients with proved CVS.
MATERIAL AND METHODS
Subjects enrolled in this study attended a single public hospital center for hemodialysis treatment and were followed at a single vascular access outpatient clinic from 2013 to 2018. We retrospectively reviewed all venous angiographies of prevalent patients in our hemodialysis units. In patients with venous angiography showing CVS, we evaluated history of prior short-term and long-term upper CVC and CRD insertion, as well as symptoms associated with CVS, rate of loss of vascular access for hemodialysis related to the presence of CVS and prevalence of cardiovascular risk factors such as heart failure, hypertension, dyslipidemia, diabetes, smoker status, obesity, and hyperuricemia. All patients were attended by the same multidisciplinar team that includes nephrologists, radiologists, and vascular surgeons before and after CVS diagnosis and CVC placement.
Analysis was based on retrospective data collection without any identification of patients thus not requiring specific ethics committee approval.
Statistical analysis
All data are presented as mean ± standard deviation (SD) or as percentage of the total cohort. The Shapiro-Wilk normality test was used to determine the normality of the groups. Outliers were identified using the GRUBS method (α = 0.01). Comparison between groups were done using a t-test or a Mann Whitney test, according to normality.
Statistical analysis was performed using GraphPad Prism 8.0.1 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
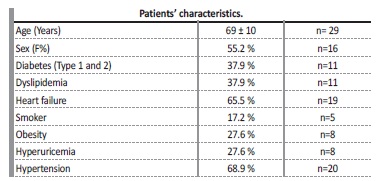
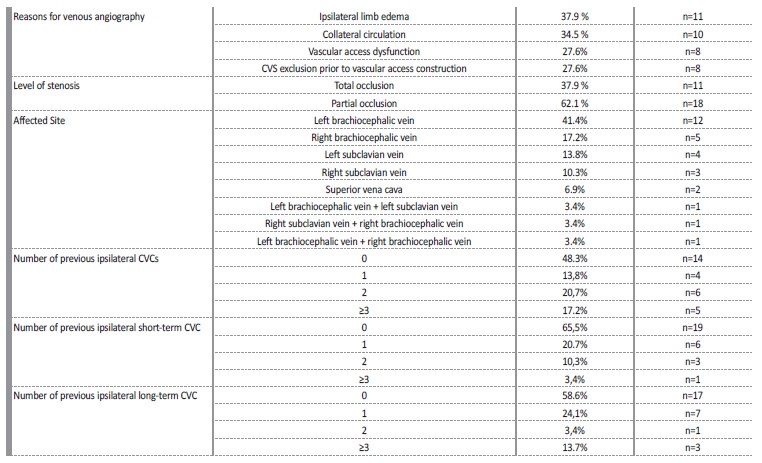
On total, 209 patients were on intermittent hemodialysis at our centre between 2013 and 2018. From these, 31 CVS were identified in 29 patients (13.8%) after undergoing venous angiography. Average patient age at time of venous angiography was 69±10 years, and percentage of female sex patients was 55% (n=16). Patients were evaluated for other cardiovascular risk factors, such as diabetic status, dyslipidemia, smoker status, obesity, hyperuricemia, and high blood pressure. The main patients’ characteristics at the time of venous angiography are summarized in Table 1. Left brachiocephalic vein (LBC) was the most affected central vascular site, in 41.4% (n=12), followed by the right brachiocephalic vein (RBC) in 17.2% (n=5), left subclavian vein (LSC) in 13.8% (n=4), right subclavian vein (RSC) in 10.3% (n=3) and superior vena cava (SVC) in 6.9% (n=2). All the stenosis in the subclavian veins were located on the transition to the homolateral brachiocephalic vein. Three patients presented more than one CVS, involving either LBC and LSV, RBC and SCV or LBC and RBC. Ipsilateral CVS was present in all patients with an history of cardiac rhythm devices (CRD) insertions (n=4, 13.7%).
Signs of venous hypertension were present in 15 patients: limb oedema was present in 11 patients (37.9%) and collateral venous circulation was present in 10 patients (34.5); 6 patients (20.7%) presented both these clinical features. In asymptomatic patients (27.6%; n=8), CVS exclusion prior to vascular access construction (protocol in all patients with an history of previous central devices) was the motive for venous angiography. In 6 patients (20.7%) CVS was accidentally found during angioplasty for other AV access problems.
Vascular access dysfunction (defined as abnormally high venous pressures during dialysis treatment, decreased access flow, increased bleeding times after dialysis or an unexpectedly low Kt/V) was presente in in 27.6% (n=8) of patients.
Most patients (75.9%, n=22) had had at least one CVC, 51.7% (n=15) had a prior ipsilateral CVC and 34.5% (n=10) had more than 1 prior ipsilateral CVC; all of these were internal jugular CVC. At least one previous ipsilateral short-term CVC was found in 34.5% (n=10) of patients and at least one previous ipsilateral long-term CVC was found in 41.4% (n=12) of patients; 48.3% (n=14) of patients had no history of previous ipsilateral CVC. Rates of patients with multiple short-term ipsilateral CVC and long-term (tunnelled) ipsilateral CVC placements are expressed in Table 2. All patients who had a prior CRD (n=4, 13.8%), had developed an ipsilateral CVS.
Average time between last ipsilateral CVC placement and CVS diagnosis was 31 ± 36 months. Total occlusion on CVS was found in 37.9% (n=11) and partial occlusion was found in 62.1% (n=18). Loss of vascular access for hemodialysis due do CVS was observed in 20.7% of all patients (n=6).
DISCUSSION
CVS is a common complication in hemodialysis patients submitted to CVC. There is substantial variation of reported frequencies of CVS among different studies, with prevalence rates ranging from 9%-12%17,18 to higher frequencies of up to 51%.8,14,19 In our study, prevalence of patients with confirmed CVS was 13.8%. From all patients with confirmed CVS, more than half (15 patients, 51.7%) had a prior ipsilateral CVC, 34.5% (10 patients) had more than 1 ipsilateral CVC and 4 patients (13.4%) had an ipsilateral CRD, making the total number of patients with ipsilateral vein manipulation19 (65.5%). Ten patients had no history of an ipsilateral CVC, but 7 of them had a previous contralateral CVC, which might still cause stenosis in the transition segments. In essence, only 3 patients (10.3%) did not have an history of previous central vein manipulation to justify stenosis. The significant prevalence of cardiovascular risk factors and heart failure in this population may help explain why some of these patients had a CVC, instead of an arteriovenous access.
While 41.4% of patients had an history of previous long-term (tunnelled) CVC, 34.4% of patients had an history of prior short-term CVC, hinting an important risk of vascular lesion even during short periods of catheterization. Past studies have shown there is a strong association between CVS and the duration of tunneled CVC dependence,3,8 but any central vein device may increase the risk of CVS.9 Studies have reported rates of CVS in non-hemodialysis patients bearing CRDs ranging from 22% to 64%, though in many cases CVS detections were incidental and patients were asymptomatic.7,20,21
In our study, all patients who had a CRD (n=4, 13.8%), had developed an ipsilateral CVS. As a multiple comorbidity population, hemodialysis patients increasingly require CRD implantations due to high prevalence of cardiovascular disease, including heart failure and arrhythmia.22 Our study upholds previous studies suggesting type and location of vascular access should be considered when an implantable CRD is needed.23
In hemodialysis patients, CVS tend to become more symptomatic as there is an increase in an extremity blood flow because of AV access. CVS symptoms occur because of AV flow and venous hypertension distal to the site of stenosis and vary according to its site.10 In our study, more than half of all patients had symptoms of CVS (only 27.6% were asymptomatic), with 37.9% of patients presenting with ipsilateral limb edema, 34.5% with collateral circulation, and 20.7% with both clinical symptoms. Vascular access dysfunction was present in 27.6% of patients. CVS exclusion prior to vascular access construction led to venous angiography in 8 patients; in 4 patients, venous angiography was performed due to findings suggesting CVS during angioplasty for other AV access problems.
Controlling risk factors for CVS should be a major focus in the predialysis and dialysis periods, as CVS contributes to significant morbidity and mortality and a considerable economic burden.3 It is desirable to avoid the placement of any CVC because it is an important risk factor for latter development of CVS. Early referral to the nephrologist with an early AV access planning and a more liberal use of peritoneal dialysis can be effective approaches to reduce catheter rates at dialysis initiation.24 In cases in which there is no other option than CVC insertion, it is important to consider the location of the catheter before placement, as subclavian CVC are known to have a greater risk of CVS than jugular and femoral ones.25 The fact that the number and duration of CVC are also risk factors for CVS occurrence should be considered.8
Finally, an AV access surveillance program may potentially allow the early detection and management of AV access problems,26 which in turn may significantly reduce the need for CVC insertion.
The major limitations of the current study are sample size and the fact that angiographies were not performed in all patients, restricting our capacity to draw conclusions about subclinical CVS.
In conclusion, there was a significant prevalence of detected CVS in our patient population. A high percentage of patients with CVS had an history of previous ipsilateral CVC. The rate of patients with previous short-term CVC was similar to that of previous long-term CVC, hinting an important role of short-term CVC as a risk factor do CVS.