INTRODUCTION
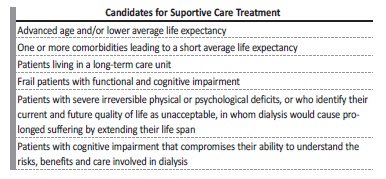
Nephrologists are dealing with an increasing number of patients with chronic kidney disease (CKD) who are older, frail, with several comorbidities, and a poor functional status. In these cases, several studies, mainly observational, showed no increase in survival and a higher risk of hospitalization upon initiating dialysis.1-8Supportive care (SC), with a “non-dialysis pathway”, may be an option to manage these patients, focusing on patient-centred goals, their quality of life (QoL) and relief from symptom burden (Table 1).3-6,9
European Renal Best Practice (ERBP) Guidelines on the management of older patients with CKD suggest evaluation of mortality risk, frailty, and CKD progression.9
Frailty is a major geriatric syndrome, with a high burden in clinical prognosis, and high prevalence in those on dialysis.10,11Early in the CKD progression trajectory, a comprehensive geriatric assessment could help to recognize frailty and other geriatric syndromes, allowing an individualized approach with early intervention that could reverse these syndromes.12
The New Zealand Guidelines suggest several tools to help with the decision SC versus dialysis, such as the ‘surprise’ question: “would you (the nephrologist) be surprised if your patient died in the next 12 months?”; nutritional status, through the Subjective Global Assessment; age (over 80 years old); comorbidities burden; and functional status.11
This extensive evaluation allows nephrologists to have an honest discussion about patients’ expectations, prognosis and QoL in dialysis, with shared decision making, to enable a truly informed choice and discussion of advance care planning (ACP).13
Strong evidence is still lacking on which models of care and interventions might be most beneficial to this population, so future research is essential.14 Meanwhile, we take the opportunity to share our experience with this case report.
CASE REPORT
We present the case of a retired 92-year-old male patient who chose SC after a shared decision-making process. He was a widower who lived alone in an apartment, with good family support. His personal medical history included dyslipidemia and benign prostatic hypertrophy. He denied tobacco or alcohol consumption. Usual medication included tamsulosin and ethyl loflazepate. At that time, he was completely autonomous for basic daily living and needed some help for instrumental activities (cleaning the house and grocery shopping). He had been monitored at our nephrology clinic due to CKD of unknown etiology since 2014. The patient presented CKD since 2004, with a creatinine of 1.36 mg/dL.
He was referred for a nephrology appointment in 2013, with a creatinine of 2.3 mg/dL and a proteinuria of 1.77 g/24 hours. During follow-up, etiological study was inconclusive: laboratory testing was negative, ultrasound showed normal size kidneys, with loss of corticomedullary differentiation and multiple bilateral cysts, contraindicating kidney biopsy. Between 2013 and 2019 kidney function slowly decreased to a creatinine of 5.44 mg/dL (eGFR of 9 mL/min/1.73 m2-CKD-EPI equation). After an informed decision-making process, he chose to be referred to the SC program. The patient was completely aware of the consequences of his decision because he had been his wife’s caregiver and responsible for her peritoneal dialysis (PD) treatments for eight years.
At the SC appointment, he was evaluated using our usual template (Table 2). He was given an initial geriatric assessment: the patient presented similar Katz and Lawton index of 6 (autonomous in basic and instrumental daily life activities, respectively); Karnofsky index of 70%; Charlson Comorbidity Index of 7; a Canadian Frailty score of 3 (managing well), Edmonton Frailty Scale 6/17 (vulnerable); depression screening was negative (2 points in Yesavage scale); and the gait evaluation did not show relevant problems (Timed Up and Go test <12 seconds). On objective examination he was lucid, with a blood pressure of 144/66 mmHg, a heart rate of 86 bpm, and no signs of respiratory distress or edema of the limbs. During follow-up, the main concerns were pruritus, sarcopenia, and fatigue. A dermatologist prescribed antihistamines, which offered only slight improvement of pruritus. A nutritional assessment was performed, and he started food supplements with adequate nutritional value for CKD (low protein so as not to aggravate acidosis or nitrogen retention). Fatigue associated with CKD anemia was managed with erythropoiesis stimulator initiation. Hyperparathyroidism was controlled with vitamin D cholecalciferol supplementation, and hyperkalemia treated with potassium binder. The proteinuria/creatinuria ratio remained stable between 1-2 g/day and was not treated pharmacologically because the patient never presented nephrotic syndrome and had already presented hyperkalemia with poor tolerance to a potassium binder due to constipation (patiromer was not yet available). He kept the vaccination plan up to date. Good family support was ensured, with the daughter-in-law as the reference relative, and his son’s support.
ACP of the patient’s wishes and goals of care were discussed in a timely manner and the patient stated his wish to remain at home until death with support from the SC team. In November 2021 he had a fall during the night, leading to a worsening clinical status and laboratory values. Home hospitalization was proposed, and both patient and family accepted. His laboratory values did not improve, suggesting he was nearing the end of his life. This situation was explained to his family who knew the patient’s goals of care. He died at home with the support of his family and his wishes had been followed in the SC program for two and a half years with a good QoL, in his own words.
DISCUSSION
Nephrology paradigm has been challenged by an older and frail dialysis population, with QoL as important as lifespan. There is growing evidence proving that SC allows an approach that prioritizes patientcentred management instead of disease-centred management.10,11
SC is a “non-dialysis pathway”, that applies knowledge of palliative care in parallel with managing CKD specificities. This approach emerged once it was found that, for some patients, dialysis does not increase survival or QoL.5,14,15 The survival of patients on SC is variable and depends mainly on frailty.6 An observational study by Carson R et al16 evaluated 202 patients ≥70 years with end-stage-kidney disease (ESKD), who chose either kidney replacement therapy (KRT) or SC. The first group included elderly patients with significant comorbidity, who experienced an increased survival of 2 years on dialysis, while the second group survived a similar number of hospital-free days compared to patients who chose hemodialysis.
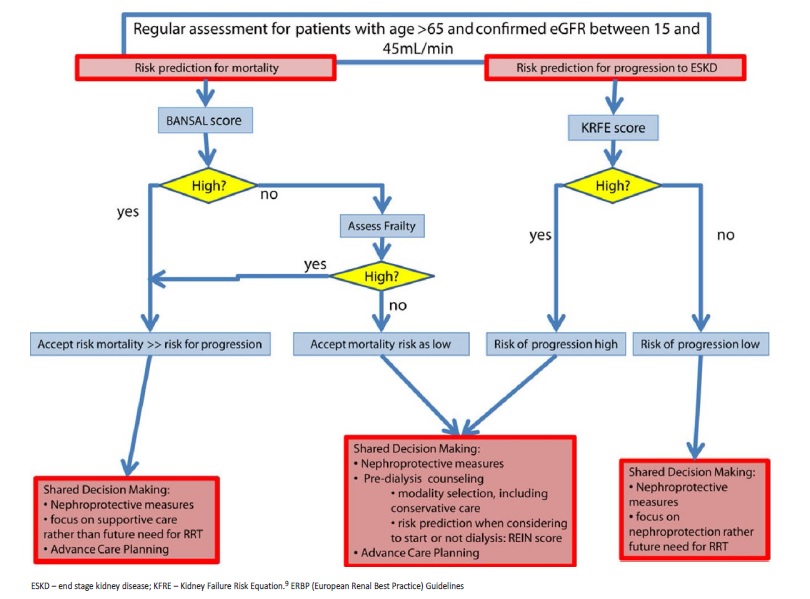
The ERBP guidelines on management of CKD older patients recommend an initial assessment to estimate CKD progression and mortality risk, providing an insight into these decisions (Fig. 1).9 Bansal score is suggested for mortality risk assessment. In those with a low mortality risk, frailty evaluation should be performed. REIN score is proposed to estimate short-term mortality risk after dialysis initiation. In patients with a low mortality score, KFRE (Kidney Failure Risk Equation) should be used to estimate CKD risk of progression (Fig. 1).7,9
In this case we present an older autonomous man, with good family support. According to the ERBP algorithm, the best approach for our patient was a shared decision-making process, focusing on nephroprotective measures and supportive care, rather than preparing for dialysis.9,17 KRFE was unnecessary since the patient was already at stage 5 CKD, with sarcopenia, hyperkalemia, anemia and secondary hyperparathyroidism. Pruritus and fatigue were the CKD-related symptoms that could interfere with his QoL. Our patient presented a high risk of mortality (93.7% risk of death within 5 years- Bansal score).
Other tools that could help with this decision - the surprise question, nutritional status, age (over 80 years old), comorbidities burden and functional status -, were also used. In line with these tools, our patient was an older man with few comorbidities and a high functional status.
He presented sarcopenia, so his nutritional status required improvement. Regarding the surprise question, the answer would be “No, I wouldn´t be surprised if he died within 12 months”: despite the interventions to improve global status and frailty, he was still 92 years old.
Nephroprotective measures should be a priority for all patients, especially in the earlier CKD stages.8,18It may include antihypertensive and antiproteinuric drugs, supplemental bicarbonate, and eviction of nephrotoxic agents. Erythropoiesis-stimulating agents should be used to correct anemia until symptoms improvement, and iron status must be checked. Ion exchange resins or patiromer could be used for hyperkalemia. For pruritus, non-pharmacological measures should be prescribed, such us skin hydration. If non-pharmacological treatment is ineffective and pruritus is directly related to uremic elevated blood level, small doses of gabapentin or pregabalin can be considered.
Secondary hyperparathyroidism and hyperphosphatemia should be controlled. Sarcopenia requires nutritional intervention and rehabilitation.18 To achieve guidelines analytical targets is not the SC objective at all.
In summary, the main aims of all prescriptions were to:
• decelerate progression of kidney disease;
• manage symptoms directly or indirectly related to CKD.
We believe that a successful SC program requires a structured approach, with a timely referral, and specific resources in this area.
In our hospital, all nephrologists can refer a patient to the SC program: first, the patient must attend a multidisciplinary appointment about ESKD treatment options; if SC is the modality that encounters patients’ goals of care, the referral must be made. Our team carries the followup in the SC program, mostly with a doctor and a nurse, with a frequency of one or each 2 months. A direct hospital contact is provided to facilitate communication.
The SC team understands that symptoms’ control, QoL, and hospital-free days are as important for patients and families than survival, and this represents a major concern on SC practice.18,19 Patient wellbeing is paramount to any decision. Moreover, the medical appointment includes ACP and written advanced directives must be discussed.18 Specific cases require palliative care expertise and end of life care. This treatment plan is individualized for each patient and may require modifications over time, especially to avoid inappropriate interventions.18 Palliative care for SC patients is established directly by the Nephrologist, who is part of the Palliative Care Program of our hospital. After evaluation by Palliative Care team, home care or referral to a palliative care unit can be provided.
At the timing of ESKD treatment choice, patient’s perception about the option which is being proposed is crucial. Not all patients understand the dialysis treatment concessions; some regretted choosing dialysis, reporting as their initial motivation the desire to please their doctors or family members.20,21 With our patient this was not a concern since, because he has been his wife´s PD caregiver, and he knew that SC suited best his goals of care.
At our hospital, we use a person-based approach (Table 2).18 The first phase always involves assessing the patient, by collecting personal history and objective health data. To evaluate frailty, we use the Edmonton Frailty Scale that includes functional parameters as basic and instrumental activities, Timed Up and go Test (to evaluate gait), medication or previous inpatient records. An evaluation of the patient’s cognitive capacity (Clock drawing test) and mood (Yesavage score) is also performed. For comorbidities we use the Charlson comorbidities Index. We use scales to access the patient’s degree of autonomy and independence, but also to identify specific areas to guide our interventions.20 Two parameters are given special attention: the symptoms evaluation, through the Renal Positive Outcome
Scale, and social problems (evaluation of cohabitants, potential caregivers, home, and possible access to complex palliative care). Expectations are evaluated and preconceptions regarding the appointment are demystified and the objectives approach to CKD complications are discussed. Therefore, SC must include clinical features as required for any nephrology appointment, but with the aim of symptom management and with the focus on a decision-making approach, and assistance with ACP.2,3,9,18,22
We use prognosis scores to guide ACP conversations. However, most widely known scores, such as the REIN score,17 are not routinely applied. With this score, our patient would have high probability of death at six months after initiating dialysis (21%). However, he lived 30 months without dialysis, with a good QoL.
The major interest of this particular case lies in the discussion surrounding the best approach for patients who choose not to start KRT. Despite his advanced age, after assessment, we realized he presented a low level of comorbidities, low six-month mortality risk and had a good autonomy degree. At this stage, nephrologists could not rule out the possibility of starting dialysis. This option was duly discussed with the patient and close relatives. Unlike most patients who receive information about the different approaches, this patient was already familiar with PD, an interesting technique for a patient who, besides being familiar, already knew how to perform the treatment. However, he refused to start any dialysis. The patient maintained his QoL and autonomy until the end.
A specialization in SC enables to recognise patients who most benefit from this modality with a balance approach between patients’ survival, best QoL possible, and lowest disease burden. Two systematic reviews of SC outcomes, recently published, showed that median survival varied according to different parameters, including geographics and local approaches to care.25,26 This makes us realize the impact of our SC knowledge and practice on patients’ lives. Nonetheless, studies are substantially heterogeneous and consistent approaches need to be established.
His wishes regarding end-of-life care were respected, and he died at home having received home medical support in the last days of his life.
CONCLUSION
There has been an increasing interest in recent years in survival benefit offered by KRT to elderly patients with ESKD and significant comorbidities, compared to SC. However, little information on patient outcomes is available and more important than that, survival does not necessarily reflect the most important concerns to patients and their families.17,25,26
The authors intend to remind that there is always a “non-dialysis” option for patients with ESKD. Knowledge in SC may be the answer to underrecognize and undertreat CKD related symptoms.
The best models of care are not yet available, but therapeutic individualization, informed decision-making, and the establishment of a plan based on the patient’s values seem to bring greater comfort to patients.2 Kidney SC will remain a way to help reduce suffering, manage the burden of CKD and validate human experience of illness.23,24