INTRODUCTION
A growing problem in the field of kidney transplantation is the accumulation of highly sensitized patients on the transplant waiting list,1,2 which is associated with high mortality rates on dialysis.3,4 Therefore, extra effort needs to be taken to transplant this group of patients. These patients develop antibodies against nearly all common human leucocyte antigen (HLA) types, present in the donor population, making transplantation of cross-match-negative organs very difficult. Sensitization toward HLA can occur through pregnancies, blood transfusions, and prior transplants. Since the presence of pretransplant donor-specific antibodies (DSAs) is associated with highest risk of immune-mediated rejection, potential donors with HLA antigens that induce a reaction with antibodies produced by the patient (unacceptable mismatches), are usually excluded. Commonly, patients are regarded as highly sensitized when having an HLA antibody profile that reacts to ≥ 80%-100% of the donor population, usually expressed as calculated panel reactive antibody PRA (cPRA).5
We present the case of a 34-year-old hypersensitized female who underwent renal retransplantation after reclassification of unacceptable antigens based on HLA epitope analysis.
CASE REPORT
We report a 28-year-old woman with end-stage renal disease of undetermined aetiology, that was admitted for living-donor kidney transplant in 2016. She was on peritoneal dialysis for 1 year before undergoing her first kidney transplant. Her immediate post-transplant course was complicated with arterial graft thrombosis and allograft nephrectomy. During this hospital stay, there was the need of blood transfusions. She resumed peritoneal dialysis after only one-month post-transplant, when her immunosuppression was weaned.
She was referred to our centre in 2020 for evaluation for a potential second kidney transplant. Her other medical problems include hypertension without significant end-organ damage. No history of pregnancy or other sensitizing events other than those mentioned.
Her physical examination was unremarkable. She had no more potential living donors. After completing the pretransplant routine evaluation, this candidate was placed on the active waiting list for deceased donor transplant.
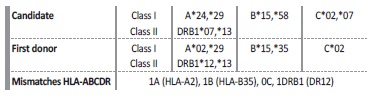
Patient HLA typing was performed using LabType™ rSSO kits according to the manufacturer’s instructions (One Lambda, Inc). Candidate and first donor HLA typing are presented in Table 1.
To determinate the specificity of the HLA antibodies, a singleantigen bead (SAB) assay (LabScreen Single Antigen Beads®, OneLambda, inc) was performed in the patient. The median fluorescence intensity (MFI) of each bead was measured using FEXMAP 3DTM flow analyzer (Luminex®, Austin, TX, USA). The analysis was performed using HLA fusion® software (v-4.6, One Lambda Inc.) and a cut-off for a positive reaction was set at MFI value of ≥1000. Several anti-HLA antibodies were identified (Fig. 1). We used the Eurotransplant Reference Laboratory (ETRL) virtual PRA calculator (https://www.etrl.org/vPRA.aspx) to obtain the cPRA, which was 99.53%.
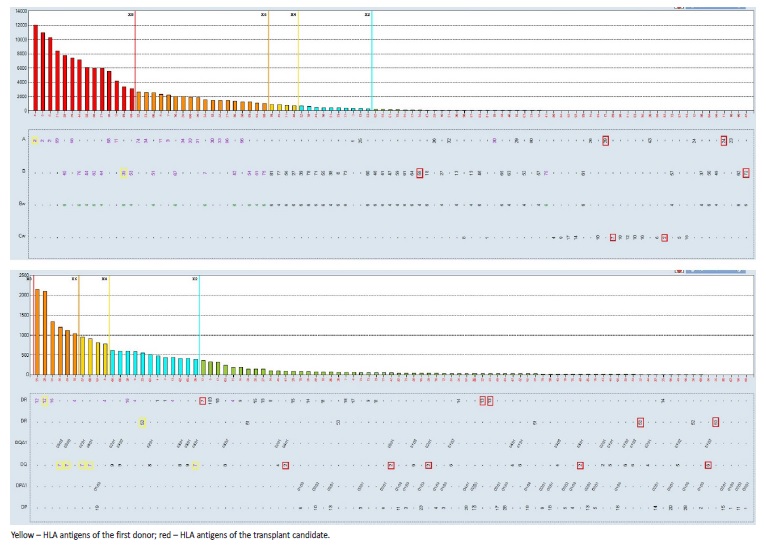
Figure 1 Analysis of HLA antibodies present in serum’s patient (16-12-2016) with Luminex SAB assays.
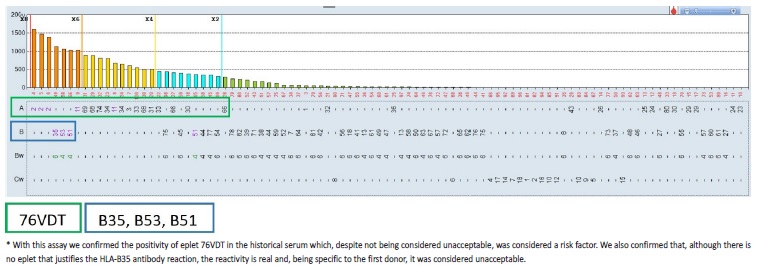
Given the high cPRA and, consequently, the low probability of finding a compatible deceased donor for our candidate, we used the HLAMatchmaker within the HLA Fusion® software to determine which eplet(s) may be responsible for the observed antibody reactivity patterns (Fig. 2). We also performed adsorption and elution technique using a cell expressing HLA-B35, and tested the eluate using SAB assays, mainly to assess the importance of HLA-B35 antibody, specific to the first donor, but not explained by HLA eplet analyses (Fig. 2). The unacceptable donor HLA antigens were re-defined: DR12 DQ7 DQ8 DQ9 A2 A68 A69 B35 B44 B45 B76 B82 B53 B51. Considering only the re-defined unacceptable antigens listed, cPRA decreased to 88.38%. One month after this unacceptable antigen reclassification, the patient was admitted for brain-dead deceased-donor kidney retransplantation.
The donor was a 21-year-old male with a subarachnoid haemorrhage as cause of death. The ABO blood type of the donor and recipient was compatible. Five HLA antigens were mismatched considering HLA-ABDR loci: 1A, 2B, 2DR. HLA typing donor performed by real time PCR (LinqSeq HLA 11 loci 384 kit, One Lambda) was: HLA-A*03,*30; B*14,*18; C*05,*08; DRB1*01,*03; DRB3*02:02; DQB1*02,*05; DQA1*01,*05; DPB1*02:02,*04:01; DPA1*01. The virtual crossmatch was positive (DRB3*02:02 with 1023 MFI) but complemente dependent cytotoxicity crossmatch was negative and flow cytometry crossmatch (FXCM) for B-cells and T-cells was also negative.
The FXCM with historical serum was positive for T cells (channel median deviation of 88, positive if ≥50), and negative for B cells (channel median deviation of 25, positive if ≥80). After evaluation, kidney transplant was proceeded. Surgery elapsed without intra-operative complications. Induction immunosuppressive treatment included methylprednisolone, thymoglobulin (5 mg/kg for 6 days) and mycophenolate mofetil (started at a dose of 750 mg/day).
Additionally, she was treated with one dose of rituximab 375 mg/m2 and a high dose of intravenous immunoglobulin (IVIG) (2 g/kg). She received a standard maintenance immunosuppressive regimen with mycophenolate mofetil, prednisone and tacrolimus 0.1 mg/kg/day, whose dose was adjusted to maintain a trough level in whole blood between 8 and 12 ng/mL during the first month.
The graft function was delayed, and patient required dialysis on the first post-transplant day. Repeated testing using Luminex® SAB assay was performed on the serum samples at day 5 post transplantation.
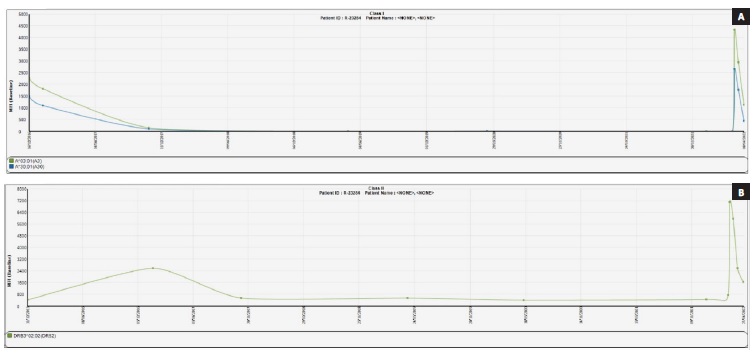
Three DSAs were detected: against HLA-A3 e HLA-A30 (with a MFI < 4000) and against HLA-DRB3*02:02 (with a MFI of 7500). Plasmapheresis was performed to reduce the levels of circulating anti-HLA antibodies (4 sessions every other day). Two days after, renal allograft biopsy was performed and revealed only mild acute tubular necrosis with a negative C4d. We repeated testing using Luminex® SAB and observed a decrease of DSAs MFI value (Fig. 3). The patient was discharged on the 22nd day with a creatinine of 1.5 mg/dL.
Four months post-transplant the patient remained with normal graft function (creatinine 1.1 mg/dL) without proteinuria. She is receiving a standard maintenance immunosuppression regime with prednisolone, mycophenolate mofetil and tacrolimus. Serial collections of patient serum have been taken for alloantibody screening using Luminex SAB assay. The DSAs MFI values continue in a descending profile and no new alloantibodies have been identified.
DISCUSSION
The techniques used to determine the degree of HLA sensitization have evolved from complement-dependent cytotoxicity assays to more sensitive techniques, including flow cytometry and solid-phase assays.
With the advent of highly sensitive bead-based luminex assays, the definition of unacceptable antigens for highly sensitized patients becomes very dependent on the interpretation and expertise of the individual laboratories. The output of luminex SAB assays is MFI for the individual beads, which is a very sensitive, but crude measure of antibody level and/or binding strength. For proper interpretation, it is of importance to realize that many factors affect the interpretation of these assays such as prozone effect, antibody titre, antibody affinity, competition for shared epitopes on different beads, complement factos and immune complex interference, as well as irrelevant antibody reactivity against denatured HLA molecules.6,7 By taking account every luminex signal, many patients end up being considered highly sensitized, with the accompanying lower chance on an organ offer through regular allocation. Therefore, it is advised a per-patient risk assessment with an individualized threshold for positivity, correlation to previous sensitizing events, HLA epitope analysis, and considering the chance of receiving a transplant.4,8
The targets for antibodies directed against HLA molecule are known as epitopes. An epitope typically, but not always, consists of a three amino acid sequence exposed on the exterior of an HLA molecule.
Each one of these molecules has multiple antibody-binding sites and different polymorphisms of the HLA molecule may share epitopes.
These shared epitopes between HLA molecules are the basis of the cross-reactive groups, whereby development of antibodies against one HLA molecule can result in reactivity against multiple HLA types.9,10 It is important to realize that alleles sharing an epitope to which antibodies have been formed, are not automatically all unacceptable since multiple contact sites determine the binding strength and thus biological function and pathogenicity of an antibody, which may differ between reactive alleles.11-13 The determination of the immunogenicity of individual epitopes is crucial, to avoid the denial of suitable organs based only on HLA incompatibility, that are clinically not pathogenic.
The most common method of predicting epitopes is using the HLAMatchmaker molecular-based computer algorithm, which is based upon three-dimensional modelling to identify exposed, polymorphic peptides present on different HLA alleles. Earlier versions used triplets; continuous linear sequences of polymorphic amino acid residues that represent the motifs of the epitope considered to be immunogenic.
More recent versions incorporate longer and often discontinuous sequences of polymorphic amino acid residues in antibody-accessible positions known as eplets.4,5
To this moment, there have been several approaches to define immunogenicity of individual eplets, each with their strengths and limitations. Identification of immunogenic eplets is possible using monoclonal antibodies directed at specific HLA molecules or using antibody adsorption and elution techniques of recombinant single HLA antigen-expressing cell lines, with alloantibodies verified using SAB assays. There is no consensus as to which specific techniques best identify and confirm the presence of antibody-verified epitopes or eplets. Evidence pertaining to the test performances and validation of these techniques as well as determination of the cost-effectiveness and practicability of the techniques are essential to future practices.7,11-14
Given the recognition that the eplet specificity analysis can provide a comprehensive assessment of HLA compatibility, independent of time and MFI threshold for HLA antibody detection,15-18 and in view of the scenario of near untranspantability, analysis of antibody profiles based on eplet specificity analysis was preceded. This evaluation was based on identification of eplets that are targeted by alloantibody present in the patients’ serum and on the identification of antigens that share amino acid sequences with this eplets but with no corresponding antibodies (unacceptable HLA antigens). This approach allowed us to redefine the donor’s potential unacceptable antigens and, consequently, reduce the cPRA significantly, increasing the likelihood of our patient receiving a kidney transplant with an acceptable match in a reasonable time frame.
A growing body of work has highlighted the association between a greater number of eplet mismatches and adverse allograft outcomes and approaches using eplet matching have been successfully implemented in organ allocation programs.5 The Eurotransplant Acceptable Mismatch program, a special program for highly sensitized kidney transplant, that has been running for almost 30 years, is based on the positive identification of acceptable antigens.3,19 These are defined as HLA antigens to which the patient has produced no antibodies, as proven by extensive laboratory testing, using Luminex SAB analysis combined with eplet analysis through HLAMatchmaker. This approach has led to significantly lower waiting times for highly sensitized patients in the Eurotransplant region, by allocating deceased donor kidneys based on a match with the recipient’s own HLA antigens in combination with predefined acceptable antigens.5,19,20 A similar program (Eurostam) has been under investigation for future implementation in the European Union.5,19,20
Although the objective is finding a compatible donor with negative crossmatch and for which the recipient has no preformed antibodies, in highly sensitized patients this may be very difficult given the large pool size needed to find a compatible match. For these candidates it is often necessary to consider transplantation across HLA barrier, with personalized immunosuppression regimens.
Desensitization protocols using the combination of plasmapheresis (PP) or immunoadsorption, to remove DSAs and/or IVIG, and rituximab to down-regulate antibody-mediated immune responses, have been proposed for these patients to address the immunological risk. Studies demonstrated a significant survival benefit among sensitized patients who underwent desensitization and transplantation compared to patients waiting for a compatible organ.21,22
These strategies are best described for patients with incompatible living donors and are applied a few days before transplantation. For deceased-donor recipient, it has been proposed that it is more sustainable to use intensive immunosuppressive therapy post transplantation rather than conducting desensitization protocols before transplantation.
Studies reporting on kidney recipients who were treated with an intensive immunosuppressive therapy starting at day 0 show acceptable short-term patient and graft outcomes.23-26 However, acute antibody-mediated rejection (AMR) continued to be an important barrier seen in 20%-30% of patients receiving desensitization protocols and these patients are at higher risk for development of transplant glomerulopathy and chronic AMR.24,26 It is still not clear which protocol (high-dose IVIG, PP/low-dose IVIG), what type of based-induction treatment (thymoglobulin, basiliximab, alemtuzumab), or if addition of rituximab is better for the prevention of early acute AMR. The crossmatch results and determining the strength of DSAs before transplantation are useful risk stratification tolls to decide the type of immunosuppression regimens.
The advantage of the intensive immunosuppression approach (as opposed to simply waiting for a better match on the waiting list) is the possibility of shortening the waiting time on dialysis. The disadvantages, however, are many, including higher costs, increased risk of infections and complications related to the higher intensity of immunosuppression, and known deleterious effects on the graft. But for some patients, this may be their best option.
Given the high immunologic risk, we decided to institute thymoglobulin-based induction immunosuppression. Since the presence of DSA by SAB assay and FXCM positive for T cells in the historical serum, we decided to add high-dose IVIG and Rituximab to the immunosuppression regimen to prevent the emergence of high levels of DSA and to decrease the risk of AMR.
These approaches require close immunologic monitoring and a low threshold for kidney biopsy to allow the early recognition of AMR manifestations. In our case, the persistence of DSA with MFI in na ascending profile was verified and plasmapheresis was instituted. The allograft biopsy revealed no manifestations of AMR.
In conclusion, the approach to the highly sensitized kidney transplant candidate must be individualized and requires careful discussion among the transplant center, patient, and histocompatibility laboratory.
The new histocompatibility and immunogenetics methodologies provide a more affirmative and comprehensive assessment of mismatch acceptability. This case highlights the importance of including these methodologies in the evaluation of the renal transplant candidate, particularly in the hypersensitized.