INTRODUCTION
Peritoneal dialysis (PD) remains an effective treatment modality for end-stage kidney disease and its use vary according to the regions. Peritonitis remains as one of the main complications of PD and one of the main reasons why this treatment is substituted by hemodialysis. It also accounts for considerable mortality and hospitalization among PD patients. Most cases of peritonitis related to PD result from the contamination caused by the poor management of the Tenckhoff catheter by the patient or care-provider. The most frequently associated agents are coagulase-negative Staphylococcus and Staphylococcus aureus. Antibiotherapy in PD related peritonitis is usually administered intraperitoneal, as it is the best way to achieve high concentrations at the site of infection.1
CASE REPORT
We report a case of an 86-year-old female patient with arterial hypertension, congestive chronic heart failure, aortic stenosis submitted to transcatheter aortic valve replacement and end-stage renal disease secondary to hypertensive nephroangiosclerosis on continuous ambulatory peritoneal dialysis (CAPD) for five years. She was regularly followed without any registration of major complications and good dialysis efficacy with 4 exchanges per day with 2 litter inflow volume for each exchange (2x physioneal 2.27% solution for 4 hours (7 hours and 19 hours) and 2x icodextrin 7.5% for 8 hours (11 hours and 23 hours). Her residual diuresis was < 100 mL/24 hours. She presented to the peritoneal dialysis (PD) unit with a 1-day history of intense diffused abdominal pain. On physical examination, blood pressure was 164/92 mmHg, heart rate was 80/minutes, the tympanic temperature was 36.5ºC, chest auscultation was normal, and the abdómen was globally painful on palpation with signs of peritoneal irritation. Catheter exit-site skin had no signs of erythema or purulent discharge.
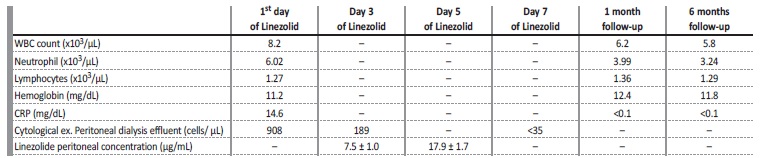
The peritoneal drainage fluid was cloudy in appearance, with 1901/mm3 cells, and predominance of polymorphonuclear cells. Complete blood count revealed 7700/uL leukocytes with 5670/μL neutrophils and 1210/μL lymphocytes. Blood chemistry showed normal hepatic function and pancreatic enzymes and elevated C-reactive protein (CRP) of 133 mg/L. Urgent abdominal computed tomography excluded secondary peritonitis, such as perforation of a hollow viscus or intra-abdominal collections. The patient was started on empiric antibiotic with intraperitoneal gentamicin and cefazolin after a single intravenous dose of gentamicin 80 mg and cefazolin 2000 mg. Peritoneal dialysis prescription was changed to 5 exchanges per day with 2 L inflow volume for each exchange (4x physioneal 1.36% solution for 4 hours (7 hours, 11 hours, 15 hours and 19 hours) and 1x icodextrin 7.5% for 8 hours (23 hours). Effluent culture revealed a hominis only sensible to vancomycin and linezolid. However, patient had a history of vancomycin hypersensitivity reaction (urticarial and generalized pruritus), so oral linezolid 600 mg (10 mg/kg) twice daily was initiated. On the 3rd and 5th days of antibiotherapy, a sample of dialyzed liquid was collected from a physioneal 1.36% solution after 4 hours its infusion, for evaluation of the linezolid concentration in the dialysate. Linezolid peritoneal fluid concentration was 7.5 ± 1.0 and 17.9 ± 1.7 μg/mL (3rd and 5th day of antibiotherapy respectively - reference values between 3 and 20 μg/mL). Antibiotherapy was maintained for 21 days in total.
There was a clinical and analytical (Table 1) improvement with no signs of recurrence to date (6 months of follow-up).
DISCUSSION
Linezolid was the first synthetic oxazolidinone antimicrobial agente available with bacteriostatic activity against gram-positive organisms. It inhibits the initiation step of bacterial protein synthesis at the 50S ribosome. It is available in a film-coated tablet form and an intravenous formulation. Oral linezolid has an excellent bioavailability, close to 100% and after absorption it undergoes hepatic oxidation to form the inactive metabolites before undergoing renal (about 35%) and non-renal clearance. Despite increased concentration of linezolid in patients with hepatic insufficiency and metabolite accumulation in renal insufficiency, there is no dosage reduction in any of these patients.2,3
Coagulase-negative Staphylococcus is the most common agente causing peritonitis in peritoneal dialysis patients, as they have been associated with the use of indwelling medical devices in combination with potential biofilm formation. Recent studies show that coagulase-negative Staphylococcus cause 60% of infections caused by gram-positive organisms and more than 40% of peritonitis overall.4 Staphylococcus hominis represent one of the most frequently identified isolates. It has been reported that some Staphylococcus hominis isolates are resistant to methicillin by the mecA gene. This gen encodes a penicillin-binding protein that helps form a bacterial cell wall with lower affinity for beta-lactam antibiotics.5
A method for quantifying linezolid in peritoneal fluid samples was developed and implemented. Our main reason was to ensure that the patient had therapeutic levels of linezolid at the site of infection.
A liquid chromatography equipment, model Waters AcquityTM Ultra Performance LC (Waters®, Ireland) was used. The mass spectrometer used was a triple quadrupole type, model Waters AcquityTM (Waters®, Ireland). The samples were analyzed in MRM (multiple reaction monitoring) mode, in order to improve selectivity and sensitivity. For data acquisition and processing, the MassLynx® version 4.1 software was used. In order to quantify linezolid, a calibration curve was prepared in solvent (ACN;H2O 50:50) at concentrations from 2 to 20 ng mL-1. In addition, two calibration curves were prepared on the samples from the linezolid drug (solution for perfusion). Each curve was analyzed in duplicate. No significant matrix effects (<5%) were detected.
In order to verify the solubility of linezolid in the solvent and experimental prescription errors, linezolid-independent solutions were prepared from the perfusion solution. The errors obtained were less than 15%. Linezolid recovery tests obtained were 100 ± 15%.
The initial management of peritoneal dialysis related peritonites include intraperitoneal antibiotics after collection of PD fluid.6 However, it is difficult for some patients to perform intraperitoneal antibiotic therapy, which sometimes requires hospitalization for the treatment of peritonitis, even in the absence of other severity criteria. The patient may in some cases avoid hospitalization, due to the possibility of carrying out oral antibiotic therapy and maintaining adequate concentrations at the site of infection (peritoneum).
In our unit, we have a peritonitis rate of approximately 1 episode per 25 months of treatment. There is a low rate of Staphylococcus aureus methicillin resistant therefore our unit protocol to treat peritoneal dialysis includes intraperitoneal cefazolin 15 mg/kg and gentamicin 0.6 mg/kg (if residual diuresis <100 mL/day) or ceftazidime 20 mg/kg. After reviewing the literature, we found only one other reports of PD-related peritonitis fully treated with oral linezolid 600 mg twice a day for 14 days7 and one where the patient was treated for one week with IV linezolid 600 mg twice a day and then converted to oral linezolid, with the same dose, for a further 2 weeks8 (Table 2). In both cases, peritonitis was caused by Enterococcus vancomycin-resistant and linezolid concentration in peritoneal dialysis samples were all greater than 4 ng/mL. The catheter was not removed from the patients.
Table 2: Treatment modality, antibiotherapy duration, catheter outcome of the two reported cases of peritonitis in PD patients treated with oral linezolid.

CAPD: continuous ambulatory peritoneal dialysis; IP: intraperitoneal; IV: intravenous
To our knowledge, this was the first Staphylococcus peritonitis in a peritoneal dialysis patient, successfully treated with oral linezolid and has had antibiotic levels monitored in the peritoneal dialysis effluent. Oral linezolid can be an option when the use of vancomycin is prohibited, as adequate concentrations are obtained at the peritoneal cavity.