INTRODUCTION
Pembrolizumab is an immune checkpoint inhibitor (ICI) used as a cancer therapy in patients with advanced head and neck squamous cell carcinoma, melanoma, non-small cell lung cancer, renal cell carcinoma, lymphoma and others. It is a highly selective monoclonal immunoglogulin G4-Kappa isotype antibody1 that selectively binds to the programmed cell death (PD-1) receptor expressed on the surfasse of T-cells. Consequently, it prevents the suppression of T-cell activation by inhibiting binding of programmed cell death ligand (PD-L1) produced by tumor cells to the PD-1 receptor.2-4 The incidence of immune related adverse events (irAE) in patients receiving ICIs can be as high as 59%-85%.5-7 The most commonly affected organs are skin, endocrine glands, gastrointestinal tract, lungs, and liver,8 but incidence of kidney toxicity related to pembrolizumab is rising as the use of this agent becomes more frequent. The most commonly reported kidney irAE is acute interstitial nephritis (AIN).9 As in other AIN etiologies discontinuation of the potential causative agent is the mainstay of therapy and glucocorticoids should be initiated in severe cases. However, in cancer patients, discontinuation of pembrolizumab can have serious implications for patient survival and glucocorticoid regimens have not been standardized. We present an uncommon case of biopsyproven AIN secondary to pembrolizumab in a patient with metastatic squamous cell carcinoma.
CASE REPORT
We describe a case of a 58-year-old Caucasian man referred to nephrology by oncology department.
The patient was diagnosed with metastatic squamous cell carcinoma in February 2022 following biopsy of a large ulcerated lesion that corresponded to a nodular conglomerate involving the latero-cervical ganglion chain. Immunohistochemistry was consistente with lymph node metastasis of a moderately differentiated squamous cell carcinoma (PD-L1 +; CPS 100). PET scan identified a hypermetabolic osteoblastic lesion in the upper third of the sternum. The disease was staged T0N3bMx according to TNM staging system. Primary location remained occult, and the metastatic conglomerate grew locally causing the patient to become increasingly hypotensive. Cervical computer tomography-scan revealed compression of the right carotid sinus due to local tumor invasion.
Other past medical history included a hemorrhagic stroke 6 years before with residual right-sided hemiparesis, past history of smoking (48 packs cigarettes/year; cessation 6 years earlier) and past drinking history (500 g/day; cessation 5 years earlier) without other toxic exposures. There was no history of kidney disease.
The patient was started on external beam radiotherapy (12+18 gray/4+6 fractions) and pembrolizumab (200 mg every 3 weeks) in June 2022 without determining primary tumor site. After 2 administrations he showed a reduction in cervical conglomerate diameter from 16 to 8 cm. Every chemotherapy cycle was preceded by blood tests to identify signs of organ toxicity associated with the drug. At the time of his fourth scheduled cycle, he presented with a sérum creatinine (sCr) of 10.1 mg/dL (baseline sCr: 1.1 mg/dL 3 weeks earlier).
The patient was admitted to nephrology department in August 2022 (he had been on pembrolizumab for almost 3 months). On admission the patient reported anorexia, postprandial nausea, weight loss (8 kg in the last 6 months), mild cervical pain related to the laterocervical mass and a mild rash on his chest, back and lower limbs. He denied fever, vomiting, diarrhea, visible hematic losses, abdominal or back pain, dysuria, upper respiratory symptoms, arthralgia, reduction of urine output or foamy urine. His regular medication included: pantoprazole 40 mg once daily, sertraline 100 mg once daily, atorvastatin 20 mg once daily, alprazolam 0.5 mg once daily and fentanyl 25 umg transdermal patch. No new medication had been added recently and he denied regular or occasional use of non-steroidal anti-inflammatory drugs (NSAIDs). On examination, his vital signs were stable with blood pressure of 98/65 mmHg (within the patient’s usual blood pressure values), pulse 73 beats per minute, oxygen saturation of 98% on room air and he was afebrile (axillary temperature 36.7°C). He had a maculopapular exanthema through his chest, dorsum and lower limbs. Two ulcerated lesions, measuring 0.5 cm in diameter each, were observed on the cervical lymph node conglomerate, with small amount of serous (not purulent) exudate. Neurological evaluation confirmed right-sided hemiparesis (previously reported). No peripheral oedema was presente and chest, cardiac, and abdominal examinations were unremarkable. His height and weight was 1.78 m and 64 kg, respectively (body mass index: 20.2 kg/m2). Initial workup consisted on blood work including serologic, immunologic and hematologic markers and urinalysis (spot collection) which are represented in Table 1. The patient had mild normocytic, normochromic anemia (hemoglobin 9.9 g/dL) with normal leucogram and platelet count. His sCr was 10.06 mg/dL and serum urea was 229.5 mg/dL, with mild electrolyte disturbances associated with acute kidney injury (AKI) (corrected hypocalcemia of 7.63 mg/dL, hyperkalemia of 5.5 mmo/L, hyperphosphatemia of 5.4 mg/dL). There was a moderate elevation of C-reactive protein (CRP) 68.9 mg/L. Urinalysis revealed leucocituria (20 leukocytes per high power field) without the presence of eosinophils and slightly elevated urinary protein (495 mg/g in urine protein/creatinine ratio).
Table 1 Initial diagnostic workup.
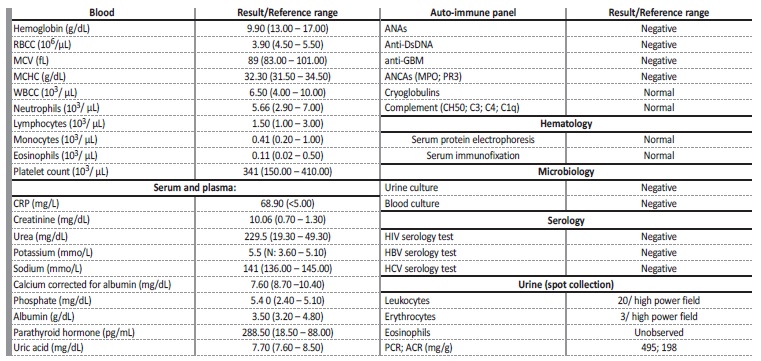
RBCC - red blood cell count; MCV - mean corpuscular volume; MCHC - mean corpuscular írus obina concentration; WBCC - white blood cell count; CRP - C-Reactive protein; ANAs - antinuclear antibodies; anti-DsDNA - anti-double stranded DNA antibody; anti-GBM - anti-glomerular basement membrane antibody; ANCAs - antineutrophilic cytoplasmic antibodies; MPO - myeloperoxidase; PR3 - proteinase 3; HIV - human immunodeficiency írus; HBV - hepatitis B írus; HCV - hepatitis C írus; PCR - protein/creatinine ratio; ACR - albumin/creatinine ratio.
Renal ultrasonography showed a slight bilateral enlargement of both kidneys with no loss of parenchymal-sinusal differentiation (Fig.1). Obstructive disease was excluded. Blood, urine, bacterial wound cultures and nasopharyngeal swab (using reverse transcription - polymerase chain reaction technique) for COVID-19 were collected on admission.
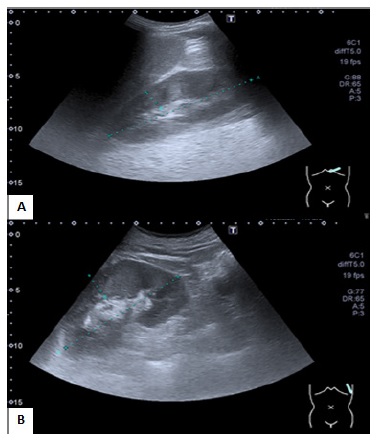
Figure 1 Ultrasound image showing renal length on right side of 125 mm with parenchymal thickness of 17 mm (A) and left side of 128 mm with parenchymal thickness of 13 mm (B).
The patient was started on intravenous fluids. Urine output was 500 mL on the first 6 hours. Pembrolizumab cycle was not administered, and given the severity of the AKI, other possible culprits of AIN were discontinued as well, namely in this patient, pantoprazole.
The patient underwent a kidney biopsy (KB) on the second day of hospitalization. The presence of na exanthema was suggestive of a systemic hypersensibility reaction. However, due to the risk of aggravating a potential bacterial infection (we had identified 2 exsudative lesions on a hypotensive patient with elevated CRP), we decided to postpone the start of corticosteroid therapy until reliminar biopsy results (optic microscopy) were available and pending microbiologic results. The patient remained apyretic with stable CRP levels and sCr levels slowly decreasing (2th day: 9.58 mg/dL; 3th day: 9.21 mg/dL; 4th day: 9.11 mg/dL). Immunologic panel was negative. KB results were available on his 5th day of hospitalization. Light microscopy showed diffuse interstitial inflammation with plasma cells and eosinophils, diffuse fibroedema, mild tubulitis suggestive of AIN (Fig. 2). No glomerular lesions were described. Vessels were unremarkable. Immunofluorescence was negative.

Figure 2 Kidney biopsy fragment on optical microscopy (×200 magnification), using haematoxylin-eosin coloration, showing mild tubulitis and interstitial inflammation with plasma cells and eosinophils suggestive of acute interstitial nephritis. The glomeruli show normal morphology.
The patient was started on IV methylprednisone 250 mg for 3 days followed by oral prednisone 30 mg/day and restarted pantoprazol. On the 10th day of hospitalization the patient had sustained, marked improvement of kidney function (sCr: 3.1 mg/dL) with normal urine output (1600 cc/day). All microbiologic results were negative. The patient was discharged with a medical re-evaluation scheduled 1 week later on nephrology department and the following month on oncology department to discuss na alternative therapy to pembrolizumab.
After discharge, the patient missed his nephrology appointment and stopped prednisolone therapy. A month later, his oncologist contacted us for a new renal function deterioration (sCr: 9.2 mg/dL). We reintroduced prednisolone (this time 40 mg/day) and planned a dose reduction of 10 mg every 5 days until a dose of 10 mg was reached, followed by a slower taper.
Unfortunately, the patient stabilized at sCr levels 3.8-4 mg/dL approximately 2.5 months after initial AKI episode. Pembrolizumab was not reintroduced for the risk of further renal function worsening and his clinical status declined due to the progression of the neoplastic disease. He was referred for palliative care.
DISCUSSION
ICIs - associated renal adverse effects have been reported as being rare,10 but their incidence is rising given the increasing frequency of immunotherapy use. A retrospective cohort study of 309 patients receiving ICIs (including 36 patients on pembrolizumab) between 2010 and 2017, demonstrated a higher incidence of AKI (16.5%), however, only 7.1% had confirmed nephrotoxicity attributed to ICIs (biopsyproven or clinical diagnosis from a nephrologist).11
ICIs-associated nephrotoxicity can be severe enough to result in established chronic kidney disease and force definitive discontinuation of the drug. The most commonly reported irAE is AIN,4 although in some series acute tubular injury (ATI)/necrosis was more prevalent.12
A few cases of glomerulopathy have been observed, mainly podocytopathy-like minimal change nephropathy/focal segmental glomerulosclerosis, immune complex glomerulonephritis, or proteinase 3 anti-neutrophil cytoplasmic auto-antibodies vasculitis.13-20
In a recent descriptive case series of patients that received an ICI and had an AKI (defined as a 1.5-fold increase in sCr) as an irAE from January 1, 2014 to December 1, 2018 at Mayo Clinic, 149 of 608 patients receiving pembrolizumab had an AKI during this period (drug-related or not), but only 7 had a biopsy-proven or clinically suspected AIN.21
In another monocentric large case series study, which included all pembrolizumab-treated cancer patients presenting a renal toxicity addressed to their center from 2015 to 2017, a total of 12 patients out of 676 pembrolizumab-treated patients were included. Patients were referred for AKI (n=10) and/or proteinuria (n=2). A KB was performed in all patients, with a median duration of use of 9 months (range 1-24 months) after the beginning of treatment. Biopsy showed that 4 patients had AIN, whereas 5 had ATI alone, 1 had minimal change disease (MCD) and ATI, and 1 had MCD alone.12
A meticulous differential diagnosis must be made in cases of AKI in patients under ICIs and renal biopsy is recommended in all cases where pembrolizumab-related AKI is suspected.22 In our case, the recent introduction of pembrolizumab, the exclusion of pre-renal, other renal and post-renal etiologies of AKI, for which complete work up was negative, and the presence of exanthema pointed to immunotherapy being the most likely cause. Time course between drug exposure and AIN is variable (2 weeks to 8 months and in some cases, extending beyond drug cessation).12
Another important aspect justifying renal biopsy is the fact that the etiology of AKI affects the decision of starting glucocorticoids. Wrongly assuming that AKI developing with an ICI is due to AIN and treat with steroids without getting a biopsy can lead to unnecessary exposure to corticosteroids without related renal function improvement.
The mechanism of tubulointerstitial injury is assumed to be similar to other drug-induced AIN.11 In pembrolizumab’s case, PD-1/PD-L1 signaling pathway interference is thought to result in loss of tolerance of selfreactive T-cells3,4,23 promoting a permissive environment for the migration of T-cell effector into the kidneys,24 which leads to an AIN pattern.
As in other cases of drug-induced AIN discontinuation of the potential causative agent is the mainstay of therapy. In our clinical case, given the severity of the AKI, pantoprazole was initially discontinued as well (although causality was unlikely). In addition to removal of the inciting agent, corticosteroids have been used to treat drug-induced AIN. Understanding their role is also important. There is no consensos about the initial dose and duration of high-dose steroid, as well as the subsequent tapering rate in drug-induced AIN.
In patients who are dialysis dependent or whose renal function fails to improve rapidly within 1 week after discontinuation of the inciting drug, some authors suggest for drug-induced AIN (not exclusively for pembrolizumab) to initiate the treatment with 500 to 1000 mg of intravenous methylprednisolone daily for three days, followed by oral prednisone at 1 mg/kg daily for definitive management of AIN.
Other authors suggest to start prednisone therapy without first giving intravenous methylprednisolone.25
In the Mayo Clinic descriptive case series study previously mentioned, they found that patients who had complete recovery of kidney function had received higher doses of steroids in the first 1-2 months (2.79 mg/kg per month; min-max, 1.45-3.2) compared with those that had a partial recovery (1.74 mg/kg per month; 0.8-3.2). Five of seven patients who received IV pulse steroids initially had complete recovery of kidney function.21 The American Society of Clinical Oncology (ASCO) guidelines recommend starting steroids for a grade 2 kidney irAE (creatinine of two to three times above baseline) and, once it improves to grade 1 or less, to start a taper over 4-6 weeks.8-26 However, Mayo Clinic study showed that a rapid taper over 4 weeks has led to rebound AKI and a slow taper over the course of at least 8-12 weeks has been better tolerated.21 Other authors have shared the same experience.25
Another recent multicenter cohort study of adults diagnosed with ICI- associated AKI between 2012 and 2020, reported no difference in the incidence or timing of recurrent AKI or death in patients treated with shorter (≤ 28 days) versus longer durations of corticosteroids.27 In our case, we faced two major challenges: the decision of what the initial dose of corticosteroids should be used, considering the lack of consensus in the literature, and the ethical dilemma of suspending an antineoplastic agent which could be the patient’s last-line treatment.
We decided to start a low dose of methylprednisolone because we were concerned about the impact on tumor response and the risk of an open wound cervical infection. We observed a good initial response to corticosteroids and a deterioration of kidney function after the patient stopping them early which highlights the importance of treatment with steroids.
Rechallenge pembrolizumab after a related AIN is another major issue. It can be a crucial treatment for a severe disease, with no other reasonable alternatives. At the same time, loss of kidney function may influence the overall prognosis. Cancer Network, suggest permanent discontinuation of ICIs for Grade 3 or 4 AKI.28 In less severe cases, some authors based on their center experience recommend that the patient recovering from an ICI-AIN be rechallenged while on low dose prednisone (10 mg/day).21
Final decision should involve collaboration of nephrologists and oncologists, adjusting for the patient’s preferences and expectations.28,29
KEY MESSAGES
1) Pembrolizumab is an immune checkpoint inhibitor (ICI) used as a cancer therapy in patients with advanced head and neck squamous cell carcinoma, melanoma, non-small cell lung cancer, renal cell carcinoma, lymphoma and others.
2) Renal adverse effects are rare with ICIs with a reported incidence up to 16.5% depending on case series. Nephrotoxicity related to pembrolizumab is rising as the use of this agent becomes more frequent.
3) The most commonly reported immune related adverse events (irAE) is acute interstitial nephritis (AIN), although in some series acute tubular injury (ATI)/necrosis was more prevalent.
4) A meticulous differential diagnosis must be made in cases of AKI in patients under ICIs and renal biopsy is recommended in all cases where pembrolizumab-related AKI is suspected.
5) As in other cases of drug-induced AIN discontinuation of the potential causative agent is the mainstay of therapy. In addition to removal of the inciting agent, corticosteroids have been used to treat drug-induced AIN.
6) There is no consensus about the initial dose and duration of high-dose steroid, as well as the subsequent tapering rate in drug-induced AIN.
7) Rechallenge pembrolizumab after a related AIN is another major issue. Final decision should involve collaboration of nephrologists and oncologists, adjusting for the patient’s preferences and expectations.















