INTRODUCTION
Although the prevalence of human immunodeficiency virus (HIV) infection remains high worldwide, there has been a significant decrease in the mortality rate of patients living with HIV. Intensive antiretroviral therapy and improved prophylaxis strategies and treatment of opportunistic infections have been responsible for these individuals to survive longer.1
Since they are living longer, patients living with HIV have begun to present, in the long term, other types of complications resulting either from the action of the virus and/or antiretroviral therapy, such as metabolic, cardiovascular, kidney and bone diseases, which are all associated with morbidity and mortality within this population.2
Bones are very often affected by HIV infection. Low bone mineral density (BMD) and decreased bone mass have been reported in these patients, regardless of age and gender. Data on fractures are limited and studies have provided contradictory data regarding the higher risk of fractures in patients living with HIV, when compared to uninfected patients.3-6Multiple factors are involved in the development of bone disease in these patients. In addition to the classic risk factors, such as aging, hypogonadism, smoking, alcohol use, nutritional status, and vitamin D deficiency, other factors related to the virus itself, its viral load and CD4 count, as well as the effects of antiretroviral therapy, also play a significant role in the pathogenesis of bone loss and fracture risk.7-9HIV acts on several pro-inflammatory pathways that interfere with the functions of the osteoblasts and osteoclasts, and, consequently, with bone remodeling.10,11Moreover, possible changes in bone metabolism resulting from the action of protease inhibitors, non-nucleoside reverse transcriptase inhibitors and nucleoside reverse transcriptase inhibitors have also been assessed in several studies.4,12,13
Chronic kidney disease (CKD) is the fourth most common cause of mortality unrelated to human immunodeficiency syndrome (AIDS) in the HIV-infected population, second only to cancer, and heart and liver diseases.14 It has been well-established that CKD causes a disorder in mineral and bone metabolism (CKD-MBD), characterized by changes in the serum levels of parathyroid hormone (PTH), fibroblast growth factor 23 (FGF-23), calcium, phosphorus, and vitamin D; by compromised bone remodeling and mineralization and by the development of vascular calcification.15 CKD-MBD is associated with the loss of bone mass and fractures, in addition to contributing to mortality, especially in dialysis patients.16
Few studies have assessed CKD-MBD in HIV patients. Therefore, taking into account the impact of CKD-MBD on the quality of life and mortality in dialysis patients and the lack of data regarding the HIV population, this study has aimed to assess the osteometabolic profile and the occurrence of vascular calcification within this population.
MATERIAL AND METHODS
A cross-sectional study was conducted at the Hospital das Clínicas at the Universidade Federal de Pernambuco (HC-UFPE), from May to October 2019, in accordance with the principles of the Declaration of Helsinki and was approved by the HC-UFPE ethics committee.
Patients were assessed who were aged over 18 years, living with HIV, and on hemodialysis in the city of Recife and metropolitan region, and were monitored at the CKD-MBD clinic at the HC-UFPE. Exclusion criteria were pregnancy, severe liver disease, presence of active infections and kidney transplantation. Of the 30 eligible patients, 21 signed an informed consent form and completed the study protocol.
All patients underwent a medical consultation, where clinical and demographic data were collected. The following parameters were evaluated: sex, age, comorbidities, time on dialysis, time since HIV diagnosis, antiretroviral therapy, medications for CKD-MBD and clinical symptoms (bone pain, muscle weakness and fractures). The presence of diabetes mellitus, systemic arterial hypertension, heart disease (defined as heart failure and/or coronary artery disease) and smoking were considered comorbidities.
The laboratory parameters analyzed were total calcium (normal range (NR): 8.5-10.5 mg/dL), phosphorus (NR: 2.5-5.6 mg/dL), intact PTH (iPTH) (NR: 15-68.3 pg/mL), total alkaline phosphatase (NR: 65-300 UI/L), 25-OH vitamin D (NR: deficiency less than or equal to 20 ng/mL; insufficiency 21 to 29 ng/mL and sufficiency: greater than 30 ng/ mL), albumin (NR: 3.5-4.8 g/dL). The exams were assessed by automation (CMD 800i; Wiener Lab Group, Rosario, Argentina). iPTH and vitamin D were analyzed by chemiluminescent immunoassay (Architect i2000 SR; Abbott Park, Illinois, USA). CD4 cell count (cells/mm3; determined by flow cytometry) and HIV viral load, determined by real-time polymerase chain reaction; Abbott Real Time HIV-1; threshold lower limit of detection of 40 copies/mL (1.60 log copies/mL) and upper limit of quantification of 10 000 000 copies/mL (7.00 log copies/mL).
In order to assess vascular calcification, x-rays of the hands, pélvis and lateral abdomen were performed, and the vascular calcification scores by Adragão17 and Kauppila18 were determined.
The BMD was evaluated by dual-energy x-ray absorptiometry (DXA) with a Lunar (Madison, Wisconsin, USA) scan (DPX-IQ). The T-score of the lumbar spine (L1-L4) and the T-score of the femoral neck were assessed, considering the definitions by the World Health Organization (WHO,1994), which are based on the standard deviation in relation to the young adult (normal: up to -1.00; osteopenia < -1 > -2.5 and osteoporosis ≤ -2.5).
Analyzes were performed using R 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria, 2019). Numeric variables are presented by mean value (standard deviation) if they follow the normal distribution, and by median (interquartile interval) if they do not. Categorical variables are represented by their respective quantities (n) and percentage (%). For the comparison between the fracture and non-fracture groups (yes/no), when categorical variables were considered, the Fisher’s exact test was used, and for numeric variables, the Student’s t test was performed for variables that followed a normal distribution, and the Wilcoxon test for non-normal variables. The Shapiro-Wilk test was used to test normality. The Spearman correlation coefficients were calculated for the variables of interest. The level of significance used was 5% (p<0.05 indicates a significant difference or significant association between variables).
RESULTS
Table 1 presents the clinical, demographic and laboratory data of the 21 patients. Patients were mostly male (81%) and the median age was 48 years. The median time on dialysis was 120 months and the median time since diagnosis of HIV infection was 132 months, and 95% of all patients were undergoing antiretroviral therapy, as described in the table. In addition, there was an undetectable HIV viral load in 90% of patients and all had good CD4 counts. With regard to comorbidities, most patients presented with arterial hypertension and heart disease, and 85% had a history of smoking. In terms of the skeletal system, bone pain was present in 11 (52%) patients, proximal muscle weakness in six (29%) and fractures were present in five (24%) patients. The main fracture site was the hip, and was observed in four patients. One other patient had a radius fracture. As may be observed, there was a frequent use of medications for CKD-MBD, whereby almost 50% of patients were taking sevelamer as a phosphate binder, around 30% were taking calcium carbonate as a phosphate binder and/or as a supplement to calcium and 42.8% of patients were taking vitamin D receptor activators (calcitriol or paricalcitol). Just one patient was undergoing treatment with calcimimetic (cinacalcet).
Table I also demonstrates the laboratory parameters. Patients presented a normal median serum calcium level. Hypocalcemia was present in 42.8% of patients and none of them was hypercalcemic.
The median phosphorus level was within the normal range for the method. The level of iPTH ranged from 86 to 1759 pg/mL, with a median of 360 pg/mL. Only 3 patients presented iPTH levels less than twice the upper limit of the method, one of whom had undergone total parathyroidectomy with autotransplantation. The total alkaline phosphatase ranged from 14 to 800 UI/L, with a median of 102 UI/L. The patients presented levels of vitamin D considered adequate.
Table 1 Demographic, clinical and laboratory characteristics of patients
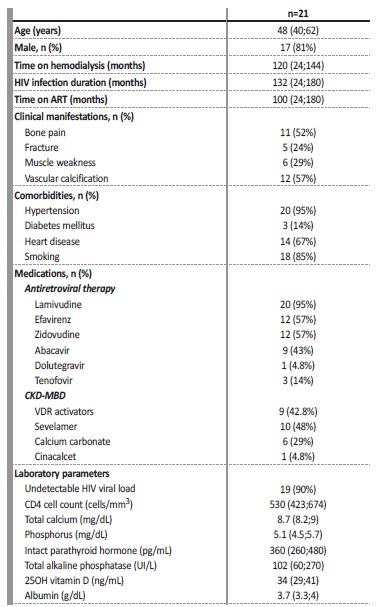
Values are expressed as number (percentage), median (25th percentile;75th percentile); ART - antirretroviral therapy; CKD-MDB - chronic kidney disease-mineral bone disorder; VDR - vitamin D receptor.
Table 2 presents the densitometry parameters and the vascular calcification scores. Based on the WHO criteria, 66.6% of patients presented a loss of BMD, including seven (33.3%) presenting with osteoporosis. Vascular calcification was observed in 12 (57%) of the patients.
Table 2 Bone mineral density and vascular calcification score
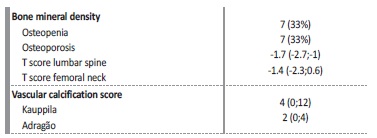
Values are expressed as number (percentage), median (25th percentile;75th percentile)
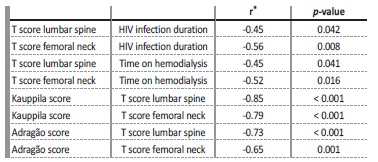
As previously described, 24% of patients had fractures. Table 3 provides a comparative analysis between the patients with and without fractures. As may be observed, the patients differed according to the Kauppila score, and was higher in the group with fractures (p=0.040). Although there was no statistical significance, the T-score values of the femoral neck (p=0.107) and of the lumbar spine (p=0.082) were much lower in patients who presented with fractures. Table 4 presents significant correlations between the clinical and densitometry parameters and between the densitometry parameters and the vascular calcification scores.
Table 3 Comparative analysis between patients with and without fractures
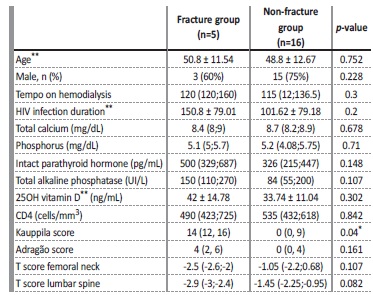
*p<0,005; ** Values are expressed as mean ± standard deviation
DISCUSSION
To the best of our knowledge, this is the first study to assess CKDMBD in patients living with HIV and undergoing hemodialysis. Our results have demonstrated a high prevalence of low BMD, fractures and vascular calcification in this population of patients.
Recent data from the Brazilian Society of Nephrology have demonstrated that, in Brazil, approximately 0.9 % of patients on dialysis live with HIV.19 Along with classic kidney disease caused by HIV (HIVAN), other kidney diseases have begun to occur in these patients, including those caused by metabolic and cardiovascular complications and those resulting from the action of antiretroviral drugs on the kidneys, thereby contributing to the development of CKD and to the increase the number of HIV patients on dialysis.20
CKD-MBD is present in practically all dialysis patients and is associated with a low quality of life and mortality in this population.15,16
On the other hand, a combination of HIV infection and antirretroviral therapy also bring about important changes in bone metabolism, which cause evident bone disease in patients with HIV. Thus, it is expected that HIV patients on dialysis carry a double risk for bone loss and fractures, in addition to other manifestations of CKD-MBD, such as vascular calcification.
Most of our patients were young male adults, who had been living with HIV and also undergoing hemodialysis for long periods of time. It is undeniable that throughout this period our patients have been exposed to several risk factors for developing bone disease. It should be highlighted that since the vast majority of patients are on antirretroviral therapy, thus enabling a better control of the HIV infection, as observed by their viral load and CD4 counts, this has contributed to both a longer survival period and a predisposition to CKD-MBD. Indeed, it was possible to observe all the manifestations in our patients that characterize CKD-MBD, from changes in the laboratory exams to the presence of vascular calcification.
The frequent use of phosphate binders, such as sevelamer and calcium salts, has probably contributed to the adequate control of the phosphorous present in our patients. We observed that the sérum calcium level, even when normal, was below 9 mg/dL and, although almost 30% of patients were taking calcium salts, hypocalcemia was still present in more than 40%. Hyperphosphatemia and hypocalcemia both contribute to the development of secondary hyperparathyroidism (SHPT). The median level of iPTH was 360 pg/mL, which was within a range considered adequate for dialysis patients, i.e., between two to nine times the upper limit for the method.15 Only 3 patients presented iPTH levels less than twice the upper limit of the method, a level considered predictive of low bone turnover.15 It is of note that the use of drugs to treat SHPT was frequent among patients, with around 43% taking vitamin D receptor activators, which may have had a positive influence on controlling the PTH. As a bone biopsy was not performed, it is not possible to determine with any accuracy which types of renal osteodystrophy are present in our patients, although the data suggest a more predominant profile of high bone turnover, such as that found in fibrous osteitis resulting from SHPT. Noe et al, studying HIV patients with normal renal function, reported SHPT in 16.9% of 1263 patients.21 These authors demonstrated that SHPT was associated with low serum levels of calcium and 25OH vitamin D, and also reported an association with the use of tenofovir disoproxil fumarate (TDF).21 Our patients did not present hypovitaminosis D, even though the median serum level (34 ng/mL) was closer to the minimum value considered adequate. We believe that in our patients, lower serum calcium levels played an important role in the development of SHPT. Other studies have also reported that the use of TDF was associated with an increase in the PTH and changes in the histomorphometry parameters of bone formation and resorption.22,23Only 14% of our patients were taking TDF and, as they are dialysis patients, we believe that the actions of this drug in bone metabolism were unimportant.
Unfortunately, there are no previous studies that have assessed CKDMBD in HIV patients on dialysis in order to be able to compare our results.
Low BMD has been reported in several studies involving patients living with HIV, occurring most significantly during the first year of virus infection and during the first two years of antiretroviral therapy initiation. Bone mass loss is even more accelerated in individuals with uncontrolled disease, such as those with CD4 counts of below24 50 cells/mm3. Brown et al, assessing the results of 11 cross-sectional studies, demonstrated a reduction in BMD of 67% and osteoporosis of 15% in HIV patients. These same authors observed that the prevalence of osteoporosis was three times higher among HIV patients when compared to non-HIV patients.4 Low BMD appears to contribute to a higher risk of fractures in HIV patients. There are several risk factors that interact and contribute to low BMD in this population. HIV patients have a high prevalence of classic risk factors for osteoporosis, such as low body mass index, smoking, alcohol abuse and gonadal insufficiency, among others.4,12,24 In addition, the state of chronic inflammation caused by the HIV virus and/or its proteins, causing an interaction between T-cells, osteoclasts and osteoblasts and an increase of inflammatory cytokines, and the effects of antirretroviral medications all play an important role in the pathogenesis of low BMD and in the occurrence of fractures in these patients.8,10,11
Our study demonstrated a high prevalence of low BMD, which was observed in 66.6% of all patients. It should be highlighted that one third of our patients were osteoporotic and that 24% of them presented a history of fractures. It is known that CKD-MBD is also strongly associated with a low BMD and with the risk of fractures, a risk which increases progressively with the loss of the glomerular filtration rate. In dialysis, fracture incidence rates range from 12 to 45/1000 patientyears.25,26 It is also known that a longer period of time on dialysis produces a greater risk of fractures, since patients are more exposed to the effects of changes in the metabolism of calcium, phosphorus, vitamin D and PTH, as demonstrated in our patients. Likewise, a longer period of HIV infection also signifies a greater risk of fractures. Indeed, we have demonstrated an inverse correlation between the T-score (of the femoral neck and of the lumbar spine) and the period of time on dialysis, in addition to an inverse correlation between the T-score of the femoral neck and the lumbar spine and the length of time living with HIV. Our patients have had long periods of time of both HIV infection and dialysis, which would justify the high prevalence of low BMD and fractures in this group.
We found no studies that specifically assessed BMD and a prevalence of fractures specifically in HIV patients undergoing hemodialysis. Unfortunately, our study did not assess a control group of non-HIV dialysis patients with similar demographic characteristics, which could have contributed to our demonstrating the role of HIV infection in bone mass. When comparing our results with other studies that assessed non-HIV dialysis patients with a similar age to our patients, we observed mixed results. Slouma et al, studying 53-year-old patients on hemodialysis for around 4 years, demonstrated a prevalence of osteopenia, osteoporosis and fractures of 45%, 23% and 12.2%, respectively.27 Khan et al, assessing patients aged 20-50 years, also on hemodialysis for a long period (more than 80 months), observed osteopenia/ osteoporosis in 16.7%/4% of the men and in 28.2/26.9% of the women.28 On the other hand, Sit et al., in patients with a mean age of 45 years, 53% of whom were men, reported low BMD in 82% of the patients, with 47% and 10% of the patients being considered osteoporotic, when evaluating the T-score in the femoral neck and the T-score in the lumbar spine, respectively.29 Indeed, in summary, the prevalence of osteoporosis and the risk of fractures in dialysis patients are high, especially when compared to the general population, although we were still surprised by the prevalence of fractures in our patients.
The impact of antiretroviral therapy on BMD and the risk of fracture in patients living with HIV has been assessed in several studies, with a variety of results. It is believed that antiretroviral therapy, with its diversity of drugs, each with a specific action, brings about changes in bone cell function, thereby determining changes in boné remodeling and consequently modifying the BMD.12,13,24 The introduction of antiretroviral therapy is associated with a decrease in BMD, which takes place during the first 6 to 12 months, ranging from 2% to 6%, in different regimens.12,30,31 Our patients had been taking antiretroviral therapy for a long period of time, with lamivudine, efavirenz, zidovudine and abacavir being the most commonly used drugs. Efavirez, through a mechanism that is still poorly defined, is associated with a deficiency of 25OH-vitamin D.32 As previously mentioned, our patients presented a normal median level of sérum 25OH-vitamin D. As also already reported, TDF was being taken by few patients. This medication may cause proximal tubulopathy, bringing on metabolic acidosis and phosphaturia, situations known to be associated with a mineralization deficit, and its use is also associated with changes in the serum level of bone remodeling markers.23
However, as our patients were on dialysis and were anuric, we believe that they were no longer exposed to these risks. Our study has insufficient data to infer the direct impact of antiretroviral therapy on BMD.
Another finding that caught our attention was the high prevalence of vascular calcification, which occurred in 57% of our patients, when assessed using the Kauppila and Adragão scores. Our results are in agreement with the literature, which demonstrates that vascular calcification is early, accelerated and highly prevalent in dialysis patients, occurring in approximately 70%-90% of patients, and is strongly associated with cardiovascular mortality.17,33,34 Vascular calcification is na active pathological process, mediated by cells and involves the media and intima layers of the arteries. Factors such as hyperphosphatemia, hypercalcemia and bone remodeling disorders (characterized by changes in the serum level of the PTH), in addition to an imbalance between proteins that inhibit and promote the calcification process, develop a central role in the pathogenesis of vascular calcification, since they contribute to the cell dedifferentiation of the smooth muscle of the vessel in an osteoblast-like cell.35 Our patients did not presente hyperphosphatemia, probably due to the frequent use of phosphate binders. However, approximately half were undergoing treatment for SHPT. The role of excess PTH in the pathogenesis of vascular calcification in CKD has been well established.36 It has also been demonstrated, specifically in HIV patients, although with normal renal function, that the PTH is harmful to vessels, and is associated with an increase in the thickness of the carotid wall.37
Several studies have already demonstrated that patients living with HIV suffer from an accelerated aging process, characterized by a higher prevalence of coronary calcification and systemic arterial calcifications and osteoporotic fractures.38,39,sss However, data on the prevalence of vascular calcification and its association with bone disease are still scarce in HIV patients on dialysis. Recently, Bellasi et al demonstrated, in patients with normal renal function, that coronary calcification was independently associated with low BMD in the femur of HIV patients.38
Our study has corroborated the results of these authors when it observed an inverse correlation between the T-score (of the femoral neck and of the lumbar spine) and the vascular calcification scores. Additionally, when we assessed the presence of fracture, we also observed that patients with fractures had a higher kauppila score when compared to those who had not suffered a fracture. Our findings demonstrate what was previously expected, i.e., in HIV patients on dialysis, vascular calcification and bone disease are also closely associated.
Our patients were considerably symptomatic since, in addition to fractures, they suffered from bone pain and muscle weakness. We believe that HIV infection and CKD play synergistic roles in the development and progression of serious complications such as low BMD and vascular calcification, which makes it difficult to measure the isolated importance of each of these pathologies.
This study presents some limitations, since it was a cross-sectional study with no control group of HIV-negative patients, in addition to having a very small sample. However, the strength of this study is the fact that it has been the first to describe the osteometabolic profile of HIV patients on dialysis. Therefore, it may become a reference for other studies to be conducted with more adequate methodologies, in order to study this serious complication of CKD in patients with such peculiar characteristics, such as those living with the HIV. In summary, HIV patients on dialysis represent a special population, since they carry with them the effects of chronic HIV infection and antiretroviral therapy on the bones, in addition to mineral and boné metabolism disorder resulting from the loss of renal function. The high prevalence of low BMD, fractures and vascular calcification observed in our patients represents an alert for the need to conduct a more rigorous assessment of bone disease in HIV patients on dialysis, so as to introduce earlier measures, such as correcting serum levels of calcium, phosphorus, vitamin D and PTH, which minimize unfavorable outcomes, such as fractures and cardiovascular mortality. Morerobust studies, preferably including bone histology, are needed to confirm our results, improve the understanding of the pathogenesis of bone disease in this population, and thereby enable treatment strategies.