Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Nascer e Crescer
versão impressa ISSN 0872-0754versão On-line ISSN 2183-9417
Nascer e Crescer vol.27 no.2 Porto jun. 2018
CASE REPORTS | CASOS CLÍNICOS
Acute Necrotizing Encephalopathy – A Rare Complication of H1N1 Infection
Encefalopatia Aguda Necrosante – Complicação Rara de Infeção por H1N1
Andreia A. MartinsI; Inês DiasII; Andrea DiasIII; Rita MoinhoIII; Carla PintoIII; Leonor CarvalhoIII
I Department of Pediatric, Hospital Pedro Hispano. Unidade Local de Saúde de Matosinhos. 4464-513 Matosinhos, Portugal. andreiaamartins87@gmail.com
II Department of Pediatric, Hospital Santo André, Centro Hospitalar de Leiria. 2410-197 Leiria, Portugal. inessrdias@gmail.com
III Department of Pediatric Intensive Care, Hospital Pediátrico de Coimbra, Centro Hospitalar Universitário de Coimbra. 3000-602 Coimbra, Portugal. sofia.andrea@gmail.com; ritamoinho@gmail.com; carla.regina.pinto@gmail.com; mleonorcarvalho@gmail.com
ABSTRACT
Acute necrotizing encephalopathy is a rare complication of H1N1 influenza infection, more prevalent in preschool children. It is characterized by a rapid neurological deterioration with progressive decline on the level of consciousness in the context of a febrile disease, associated with multifocal symmetric brain lesions.
The cases of two children (two and four years old) admitted due to an altered level of conscience in the context of febrile respiratory infection are described. The laboratory studies disclosed high aminotransferases with normal ammonia, and the presence of H1N1 virus in the nasopharyngeal swab. The brain computed tomography showed bilateral thalamic hypodensities. The clinical evolution was very distinctive in the two cases, with brain death in one and survival with good neurological outcome in the other.
The association of febrile respiratory infection and a rapid decline on the level of consciousness should be an alert to this entity, which has high morbidity and mortality. For the diagnosis, it is recommended to search for the virus in the nasopharyngeal swab. In addition to supportive treatment, oseltamivir and immunomodulators, although controversial, are the most commonly used therapies.
Keywords: Encephalopathy; Influenza A Virus H1N1 Subtype; Preschool children
RESUMO
A encefalopatia aguda necrosante é uma complicação rara da infeção pelo vírus H1N1, mais prevalente na idade pré-escolar. Caracteriza-se por um quadro neurológico rapidamente progressivo, com deterioração do estado de consciência em contexto de doença febril, associado a lesões cerebrais multifocais simétricas.
Descrevem-se os casos de duas crianças com dois e quatro anos, admitidas por alteração do estado de consciência em contexto de infeção respiratória febril (IRF). Dos exames complementares realizados, destacavam-se aminotransferases elevadas com amónia normal, presença do vírus H1N1 no aspirado nasofaríngeo (ANF) e hipodensidades talâmicas bilaterais na tomografia computorizada cerebral. A evolução foi distinta, com morte cerebral num dos casos e sobrevida com boa evolução neurológica no outro caso.
No contexto de IRF, a alteração rapidamente progressiva do estado de consciência deve alertar para esta entidade, que tem elevada morbimortalidade. Está recomendada a pesquisa do vírus no ANF para a confirmação do diagnóstico. Além do tratamento de suporte, a terapêutica com oseltamivir e imunomodeladores, ainda que controversa, é a mais utilizada.
Palavras-chave: Encefalopatia; Idade pré-escolar; Vírus da Influenza A Subtipo H1N1
INTRODUCTION
Acute necrotizing encephalopathy (ANE) is a rare and severe form of H1N1 encephalopathy.1-6 It is characterized by a rapid deterioration of the level of consciousness in association with a febrile disease and elevation of aminotransferases and normal ammonia, absence of cerebrospinal fluid pleocytosis, symmetric multifocal cerebral lesions and exclusion of other possible causes for those clinical features.1,5 The incidence is unknown, however, a growing number of cases have been reported, notably in Europe.6-8.
CLINICAL CASE 1
A two-year-old previously healthy male child, with a completed Portuguese vaccination program, was brought to Emergency Department with fever, cough and anterior rhinorrhea, developing in the previous 24 hours, associated with prostration, hypotonia and poor reactivity. At the admission, the patient presented axillary temperature of 40ºC, tachycardia with normal blood pressure and blushing oropharynx. He was somnolent but reactive without focal neurological signs; the Glasgow Coma Scale (GCS) was 13 and he had isochoric and photoreactive pupils. The laboratory results were unremarkable. One hour later, left palpebral ptosis and right hemiparesis including the lower face were detected. He was promptly started on ceftriaxone, ciprofloxacin and acyclovir, and a brain Computed Tomography (CT) scan was performed, which disclosed bilateral thalamic hypodensities, mostly on the left side (Fig.1). Three hours later, due to neurologic deterioration with GCS of eight, he was intubated and transferred by the Pediatric Interhospital Transport Team (TIP) to the Pediatric Intensive Care Unit (PICU). The laboratory tests showed normal haemoglobin, leucopenia (2700/μL) with lymphopenia (620/μL), C-reactive protein 10mg/L, normal glycemia, absence of acid-base or ionic imbalances and a slight elevation of cytolysis enzymes (AST 205UI/L, ALT 104UI/L, LDH 938 UI/L). Ammonia and coagulation studies had no changes. Cerebrospinal fluid (CSF) exam revealed normal glucose and protein levels and no pleocytosis. Whereas a clinical picture of ANE, nasopharyngeal swab was collected for molecular biology and oseltamivir was started. Due to clinical severity, he was started on intravenous 2g/kg immunoglobulin (IgG) and methylprednisolone 30mg/kg/day for three consecutive days. At day four, brain Magnetic Resonance Angiography (Angio-MRI) showed bilateral thalamic and internal capsule lesions with a central hypointensity on T2-weighted images and smaller areas of abnormal signal on left fronto-parietal, bilateral temporal and posterior areas of the midbrain (Fig. 2).
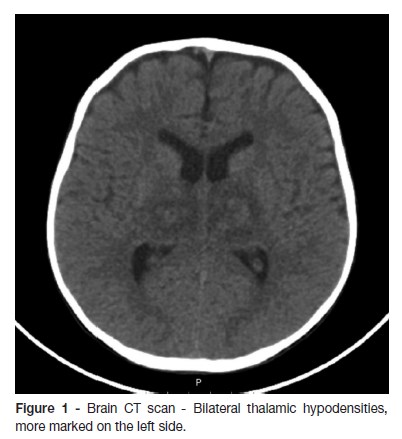
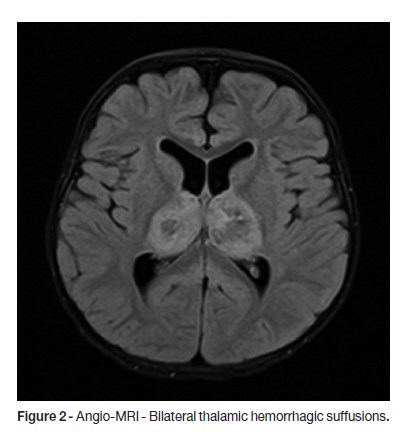
Blood cultures and the bacteriological and virological results of the CSF were negative, and serology for herpes simplex virus 1 and 2 were compatible with an old infection. Antibiotics and acyclovir were withdrawn. The identification of H1N1 by PCR in the nasopharyngeal swab corroborated the diagnosis of ANE, and five days of oseltamivir therapy was completed. He remained afebrile since day seven, when he was extubated. He was transferred to the ward at day 12, maintaining clouding of consciousness, dysarthria, poor facial mimic, left divergent intermittent strabismus, and right hemiplegia, with a good subsequent outcome.
CLINICAL CASE 2
A four-year-old female child, with Lebers congenital amaurosis, delayed psychomotor development and the Portuguese vaccination program completed, was admitted to the local hospital, presenting with a generalized tonic-clonic seizure with one hour of evolution. On her previous clinical history there was an upper respiratory tract infection during the previous week, associated with fever (maximum axillary temperature of 38ºC) for the last 24 hours. She had a tympanic temperature of 40ºC, tachycardia, increased capillary refill time and bilateral tonic-clonic movements. Therapy with rectal diazepam, intranasal midazolam, and intravenous clonazepam and phenytoin were started, with resolution of the seizure 50 minutes after admission. Treatment with ceftriaxone, acyclovir and a physiological saline bolus were also started and she was intubated. The laboratory diagnostic tests showed normal haemoglobin, leukocitosis (17200/μL) with neutrophilia (8670/μL), C-reactive protein 103mg/L, normal blood glucose, high urea (68mg/dL), normal creatinine, hypernatremia (147mEq/L), elevation of cytolysis enzymes (AST 249UI/L, ALT 95UI/L, LDH 1154 UI/L) with normal ammonia and increased activated thromboplastin time (44sec / 29sec). Urinalysis was negative for drugs and the chest X-ray was normal. She was transferred by TIP to the PICU.
At PICU admission, the patient was afebrile, with respiratory and hemodynamic stability, non-reactive sligthly asymmetryc pupils, and absent reaction to pain or cough reflex. Cerebral activity was very depressed on Integrated amplitude EEG (a-EEG). About one hour later, cardiogenic shock settled with persistent hemodynamic instability despite cardiovascular support. At the same time she developed hypothermia, central diabetes insipidus and coagulopathy. CT scan revealed bilateral symmetric hypodensities of the brainstem, cerebellum, thalami and white matter, with loss of cortical-subcortical discrimination at the left frontal-parietal, right frontal and bilateral internal parieto-occipital levels, and uncal herniation (Fig. 3). Four hours after admission, H1N1 was identified by PCR in the nasopharyngeal swab, corroborating the suspicion of ANE, and she was started on oseltamivir.
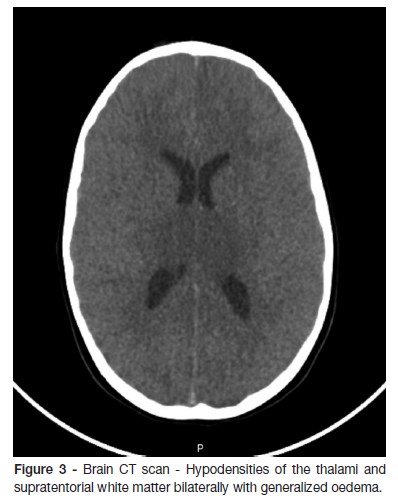
GCS of 3 remained with absence of brain stem reflexes and depressed activity on the a-EEG, evolving to an isoelectric line, without sedation. Considering the neurological features and the results on the CT scan, intracranial pressure monitoring was not performed. Brain death was confirmed by two serial examinations, and death was noticed 24 hours after admission. The autopsy revealed necrotic lesions including the pons, basal nuclei and subcortical areas, without significant lymphocytic infiltrate, changes that are characteristic of influenza encephalopathy. The cerebellum was small by destruction of the internal granular layer, likely to be related to underlying disease.
DISCUSSION
Acute necrotizing encephalopathy by H1N1 is a rare, potentially fatal neurological disorder that is more frequent in children under five years.1-11 An early diagnosis is possible through past clinical history, laboratory abnormalities and typical imaging findings, and exclusion of other etiologies such as toxic, metabolic, infectious or vascular disorders should be excluded.1-11
Typically, there is a prodromal period, characterized by fever and respiratory symptoms, on the previous 24-72 hours, followed by a rapidly progressive disturbance of consciousness, with or without epileptic seizures, that may progress to coma and, in some cases, death.1,3-5,10,11 Signs of systemic inflammatory response syndrome may be present, namely shock, multi-organ dysfunction and disseminated intravascular coagulation (case 2)..11
Laboratory abnormalities are not specific, with frequent elevations of aminotransferases with normal ammonia, absence of hypoglycemia and lactic acidosis, and high CSF protein levels without pleocytosis.1,7,8,10,11
These two case reports presented the typical, multifocal symmetric brain lesions, with a similar topographic distribution - thalami, brainstem, cerebellum and supratentorial white matter.1,7,8,11 Bilateral thalamic involvement is always present, and is a distinctive feature of ANE.11 In the brain CT scan, they present as hypodense lesions, and in MRI they appear as T1 hypointense images, with hypersignal in T2 and FLAIR, sometimes with restriction to diffusion and/or hemorrhagic transformation.10 In some severe cases, there is generalized oedema leading to brainstem herniation.5,11
ANE is usually triggered by influenza A virus, but there have been described cases caused by influenza B, herpes simplex 6, coxsackie A9, parainfluenza and mycoplasma pneumoniae.1,4,5,7,9 The H1N1 screening is done by PCR in the nasopharyngeal swabs since its identification in the CSF occurs in 10% of the cases and in the nasopharyngeal swabs by immunofluorescence it occurs in 50%.1,4,7 In the presented cases the analysis of the H1N1 virus by PCR in the nasopharyngeal swabs confirmed the diagnosis.
The pathogenesis of ANE remains unclear. It does not seem to be a direct central nervous system lesion, nor a post-infectious reaction.4,5,11 The most accepted theory is hypercytokinaemia triggered by viral infection leads to an exacerbated immune response, producing high levels of cytokines, similar to a systemic inflammatory response. This cytokine storm explains systemic symptoms such as liver dysfunction, coagulopathy and shock. In the central nervous system, cytokines, namely interleukin-6 and tumor necrosis factor alpha (TNF-α), cause vessel permeability damage, leading to cerebral oedema, hemorrhage and necrosis.1,5,11
There is no specific treatment. Intensive care, including control of intracranial hypertension, is recommended.1,7,8,11 Antiviral therapy, although controversial, is routinely used.1,7,11 Given the hypercytokinaemic environment, immunomodulatory therapy with steroids, IgG and hypothermia are therapeutical strategies.7,11 Intravenous steroids and IgG have been associated with a favorable outcome, however there is no consensus on the dosage, timing and duration of steroid therapy.7,11 Some authors consider therapeutic hypothermia as an option in the presence of cerebral oedema, especially when initiated within the first 12 hours.11,12 In the presented clinical cases, treatment with antiviral therapy in addition to supportive care was started. Immunomodulatory therapy was provided only in the first case, due to rapid deterioration in the second.
The prognosis varies widely, ranging from complete recovery in 10% of cases to severe neurological sequelae in 15%, and death in 30%.7,11
It is mandatory to consider this diagnosis facing a compatible clinical picture and bilateral thalamic lesions in the brain imaging. If the diagnosis is highly probable, early intensive support therapy, antivirals and immunomodulatory measures are recommended.
To our knowledge, these are the first two clinical cases of ANE caused by H1N1 published in Portugal.
REFERENCES
1. Martin A, Reade EP. Acute Necrotizing Encephalopathy Progressing to Brain Death in a Pediatric Patient with Novel Influenza A (H1N1) Infection. Clin Infect Dis. 2010; 50:50-2. [ Links ]
2. Gulati P, Saini L, Jawa A, Das CJ. MRI in H1N1 Encephalitis. Indian J Pediatr. 2013; 80:157-9. [ Links ]
3. Wilking AN, Elliott E, Garcia MN, Murray KO, Munoz FM. Central Nervous System Manifestations in Pediatric Patients With Influenza A H1N1 Infection During the 2009 Pandemic. Pediatr Neurol. 2014; 51:370-6. [ Links ]
4. Amin R, Ford-Jones E, Richardson SE, MacGregor D, Tellier R, Heurter H, et al. Acute Childhood Encephalitis and Encephalopathy Associated With Influenza - A Prospective 11-Year Review. Pediatr Infect Dis J. 2008; 27:390-5. [ Links ]
5. Zeng H, Quinet S, Huang W, Gan Y, Han C, He Y, et al. Clinical and MRI features of neurological complications after influenza A (H1N1) infection in critically ill children. Pediatr Radiol. 2013; 43:1182-9. [ Links ]
6. Lyon JB, Remigio C, Milligan T, Deline C. Acute necrotizing encephalopathy in a child with H1N1 influenza infection. Pediatr Radiol. 2010; 40:200-5. [ Links ]
7. Bustos BR, Andrade YF.Acute encephalopathy and brain death in a child with influenza A (H1N1) during the 2009 pandemic. Rev Chilena Infectol. 2010; 27:413-6. [ Links ]
8. Kim KJ, Park ES, Chang HJ, Suh M, Rha DW. Novel Influenza A (H1N1)-Associated Acute Necrotizing Encephalopathy: A Case Report. Ann Rehabil Med. 2013; 37:286-90. [ Links ]
9. Akins PT, Belko J, Uyeki TM, Axelrod Y, Lee KK, Silverthorn J. H1N1 Encephalitis with Malignant Edema and Review of Neurologic Complications from Influenza. Neurocrit Care. 2010; 13:396-406. [ Links ]
10. Saab S, Torres JB, Serrano S, Rodriguez N. Encefalopatía necrotizante aguda de la infancia. diagnóstico por imágenes: presentación de caso. Rev. Colomb. Radiol. 2015; 26:4223-7. [ Links ]
11. Wu X, Wu W, Pan W, Wu L, Liu K, Zhang HL. Acute Necrotizing Encephalopathy: An Underrecognized Clinicoradiologic Disorder. Mediators Inflamm. 2015; 2015:792578. [ Links ]
12. Vargas WS, Merchant S, Solomon G. Favorable outcomes in acute necrotizing encephalopathy in a child treated with hypothermia. Pediatr Neurol. 2012; 46:387-9. [ Links ]
CORRESPONDENCE TO
Andreia A. Martins
Department of Pediatric
Hospital Pedro Hispano
Unidade Local de Saúde de Matosinhos
Rua Dr. Eduardo Torres
4464-513 Senhora da Hora, Matosinhos
Email: andreiaamartins87@gmail.com
Received for publication: 11.06.2017
Accepted in revised form: 25.09.2017














