Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Nascer e Crescer
versão impressa ISSN 0872-0754versão On-line ISSN 2183-9417
Nascer e Crescer vol.28 no.1 Porto mar. 2019
https://doi.org/10.25753/BirthGrowthMJ.v28.i1.14855
ORIGINAL ARTICLES | ARTIGOS ORIGINAIS
Acute kidney injury in a pediatric intensive care unit
Lesão renal aguda numa unidade de cuidados intensivos pediátricos
João Rio MartinsI, Catarina PereiraI, Carolina AquinoII, Carla PintoI, Andrea DiasI, Leonor CarvalhoI
I - Pediatric Intensive Care Unit, Hospital Pediátrico de Coimbra, Centro Hospitalar e Universitário de Coimbra. 3000-602 Coimbra, Portugal. joaoriomartins@gmail.com; catarinaopereira@gmail.com; carla.regina.pinto@gmail.com; sofia.andrea@gmail.com; mleonorcarvalho@gmail.com
II - Faculty of Medicine, Universidade de Coimbra. 3004-504 Coimbra, Portugal. carolinarfsa@gmail.com
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Background and aims: Acute kidney injury (AKI) is a common condition in Pediatric Intensive Care setup and has been associated with increased mortality and morbidity. The aim of this study was to determine incidence, risk factors, and outcome of AKI in a Pediatric Intensive Care Unit (PICU).
Materials and methods: An exploratory study was conducted using prospective data collected from July to December 2017. Clinical and biochemical data from all patients admitted to PICU was recorded. Kidney Disease: Improving Global Outcomes (KDIGO) criteria were used for diagnosis. Patients with hospital stay shorter than 48 hours and with previously documented kidney disease were excluded.
Results: During the study period, 32 of 112 children developed AKI (28.6%). Median time of diagnosis was on the second day of admission. Overall, 59.4% of children fulfilled creatinine criteria, 68.8% urinary output criteria, and 28.1%, both. KDIGO stages 1, 2 and 3 accounted for 50.0%, 28.1%, and 21.9% of cases, respectively. Total renal function recovery occurred in 56.3% of cases, partial recovery in 28.1%, and 15.6% of cases did not recover. Invasive ventilation (p=0.028), need for vasoactive drugs (p=0.043), and shock (p=0.043) were independent risk factors for AKI. Mortality was higher in AKI (15.6%) than in non-AKI (1.3%) patients (p=0.007).
Discussion: AKI is common in PICU setting, especially in the first days of admission. Both serum creatinine and urinary output criteria should be considered for diagnosis. Use of mechanical ventilation and/or vasoactive drugs and shock are common risk factors and should elicit careful monitoring and prompt treatment.
Keywords: acute kidney injury; children; pediatric intensive care; risk factors
RESUMO
Introdução: A lesão renal aguda (LRA) é um problema frequente em cuidados intensivos pediátricos, estando associada a morbilidade e mortalidade aumentadas. O objetivo deste estudo foi caracterizar a incidência, fatores de risco e prognóstico da LRA numa Unidade de Cuidados Intensivos Pediátricos (UCIP).
Materiais e Métodos: Foi efetuado um estudo exploratório com colheita prospetiva de dados entre julho e dezembro de 2017. Foram analisados os dados clínicos e bioquímicos de todos os doentes admitidos na UCIP e aplicada a classificação Kidney Disease: Improving Global Outcomes (KDIGO). Doentes com duração de internamento inferior a 48 horas e com doença renal previamente conhecida foram excluídos do estudo.
Resultados: Durante o período de estudo, 32 de 112 crianças apresentaram LRA. A mediana de tempo de diagnóstico foi o segundo dia de admissão. No total, 59.4% dos casos cumpriram o critério de creatinina, 68.8% o critério de débito urinário e 28.1%, ambos os critérios. Os graus de KDIGO 1, 2 e 3 representaram 50.0%, 28.1% e 21.9% dos casos, respetivamente. Foi observada recuperação total em 56.3% dos casos, recuperação parcial em 21.9% e 15.6% dos casos não apresentaram recuperação da função renal. Ventilação invasiva (p=0.028), uso de inotrópicos (p=0.043) e choque (p=0.043) foram fatores de risco independentes para LRA. A mortalidade nos doentes com LRA (15.6%) foi superior à dos doentes sem LRA (1.3%; p=0.007).
Conclusão: A LRA é uma complicação comum em cuidados intensivos pediátricos, especialmente nos primeiros dias de admissão. Ambos os critérios de creatinina e débito urinário devem ser utilizados para o diagnóstico. O uso de ventilação mecânica e/ou fármacos inotrópicos e choque são fatores de risco frequentes e devem conduzir a uma monitorização cuidadosa para um diagnóstico e tratamento atempados.
Palavras-chave: lesão renal aguda; criança; cuidados intensivos pediátricos; fatores de risco
Introduction
Acute kidney injury (AKI) is a complex clinical entity with multifactorial etiology and a broad clinical spectrum. A widely accepted definition of AKI is still missing, and hence the observation of sudden onset renal dysfunction resulting from aggression by endogenous and/or exogenous mechanisms is usually adopted.1,2
According to the available literature, incidence of AKI in pediatric intensive care setup varies from 1 to 82%. It is an independent risk factor for morbidity and mortality and for progression to chronic kidney disease (CKD).3-13 Despite being considered a priority research area by the American Society of Nephrology, knowledge about AKI remains limited, especially regarding its risk factors.14
Three main validated criteria for diagnosis and classification of AKI are used in the pediatric population: pRIFLE (pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease), AKIN (Acute Kidney Injury Network) and KDIGO (Kidney Disease: Improving Global Outcomes). These criteria are based on a sudden increase in serum creatinine levels or a reduction in the glomerular filtration rate associated with a decrease in urinary output, which are late markers of renal dysfunction.4 Currently, there is a great interest in developing and studying earlier and more sensitive biomarkers of kidney injury, such as cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18), and liver- type fatty-acid-binding protein (L-FABP).15,16 However, few Centers are able to routinely use such biomarkers in daily clinical practice, making the investigation of risk factors and risk predictive models a priority clinical research area.
Although risk factors for AKI in pediatric intensive care setting described in the literature vary according to geographic area, age, need for invasive ventilation, need for vasoactive drugs, severe and generalized infection, multiorganic dysfunction, shock, and use of nephrotoxic drugs have been most frequently identified in previous studies.5,6,17,18
Research on AKI in the pediatric population is scarce, and prospective studies in pediatric intensive care are required to allow the implementation of appropriate preventive and/or therapeutic approaches able to decrease the incidence and severity of the condition.
The aim of this study was to characterize AKI in pediatric intensive care setup regarding epidemiology, incidence, demography, etiology, classification, and outcomes. A secondary goal was to identify risk factors for the development of AKI during hospitalization, as well as mortality-associated risk factors.
Materials and methods
An exploratory study was conducted using prospective data retrieved from a level III Pediatric Intensive Care Unit (PICU). Clinical and biochemical data from all children admitted between July and December 2017 were anonymized and analyzed.
Patients with a hospital stay shorter than 48 hours and those with previously documented kidney disease were excluded. For children with multiple admissions during the study period and no exclusion criterion, admissions were individually analyzed.
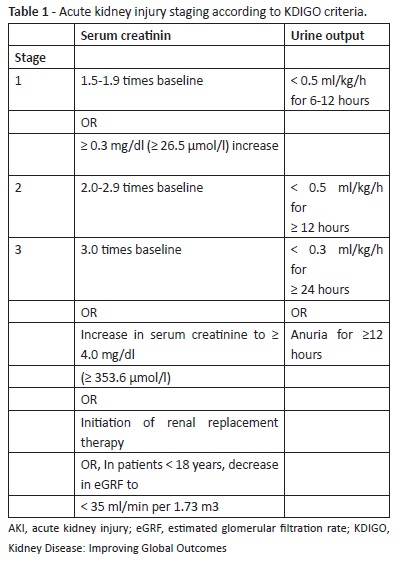
AKI diagnosis was established using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria (Table 1).19 The first value obtained was used as baseline in children with normal-for-age serum creatinine values on admission. When serum creatinine was increased on admission, the age-adjusted baseline creatinine value was estimated using the formula:
Mean Creatinine (µmol/L)age = -2.37330 - 12.91367 * Loge (age) + 23.93581 * (age)0.5
The urine output criterion was only used when a reliable quantification was obtained.
Retrieved data included demographics (age, sex, biometry); patients’ main and associated diagnoses, such as shock, trauma, multiorgan failure (MOF), rhabdomyolysis, nosocomial infection, and surgery; comorbidities; invasive techniques employed - such as renal replacement therapy and invasive mechanical ventilation − and respective duration (quantified in ventilation-free days); pharmacological treatment, including inotropics, diuretics, and nephrotoxic medication; biochemical data (creatinine, BUN, C-reactive protein, procalcitonin, blood cell count, urinalysis); estimated Pediatric Index of Mortality 3 (PIM3); and daily clinical data.
When AKI diagnosis was established, it was classified as prerenal, intrinsic, or postrenal regarding sodium fractional excretion, BUN-to- creatinine ratio, and renal ultrasound findings.
Renal function recovery was classified as total, partial, or no recovery’. Total recovery was defined as return to age-adjusted serum creatinine, blood pressure, urine output, and urinalysis values by the time of discharge from PICU. No recovery’ was defined as absence of improvement or worsening by the time of discharge or death.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS)®, 22nd edition. Absolute and relative frequencies and measures of central trend and dispersion were calculated. Student’ t or Mann-Whitney U tests were used to test for differences in quantitative variables according to their distribution after a normality test (Kolmogorov-Smirnov when sample number was >25 and Shapiro-Wilk when it was <25). Chi-square or Fisher exact tests were used to test the association between qualitative variables. Logistic regression models were fitted, using the Hosmer- Lemeshow test, to identify independent risk factors for AKI. A level of significance of 0.05 was adopted.
Results
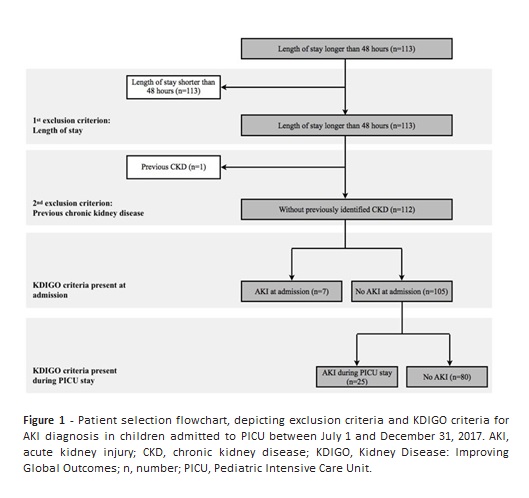
A total of 179 admissions were registered during the study period, 67 of which were excluded from the analysis for not meeting inclusion criteria: 66 patients had a hospital stay inferior to 48 hours and one patient had a previous diagnosis of CKD (Figure 1).
Among 112 children included in the study, seven had AKI on admission (6.3%) and 25 developed AKI during PICU stay (22.3%). The global incidence of AKI was 32/112 (28.6%).
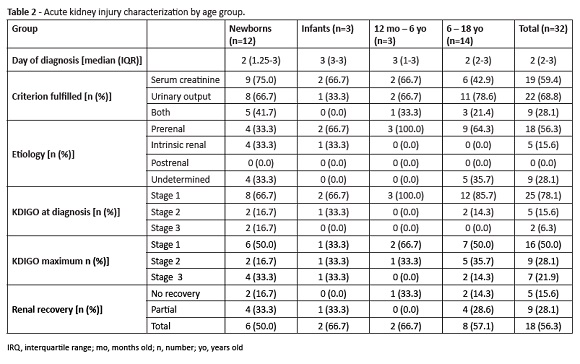
AKI incidence was 32.4% in the newborn group, 17.6% in the infant group, 60.0% in the 12 months−6-year-old group and 58.3% in the 6−18 year-old group. Features of AKI by age group are shown in Table 2.
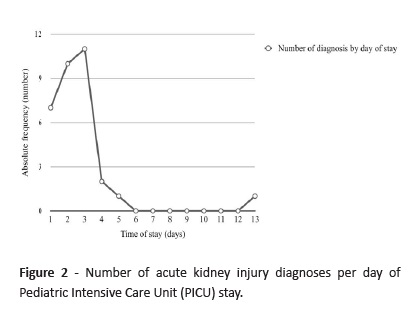
AKI diagnosis was performed until the second day of admission in 50.0% of cases and until the third day of admission in 75.0% of cases (Figure 2).
Regarding disease etiology, 56.3% of cases were due to prerenal cause and 15.6% to intrinsic renal cause. No postrenal etiology cases occurred during the study period. In 28.1% of cases, etiology remained undetermined.
According to KDIGO criteria, AKI stage at the time of diagnosis was determined as one in 25 cases for 78.1% of children, two in five cases por 15.6% of children, and three in two cases for 6.3% of children. Due to progression of renal dysfunction, nine patients (28.1%) reached stage 2 and seven patients (21.9%) reached stage 3.
Nineteen cases (59.4%) fulfilled the creatinine criterion, 22 (68.8%) the urinary output criterion, and nine children (28.1%) fulfilled both criteria. The urinary output criterion was the only found in 50.0% of stage 1 cases, 44.0% of stage 2 cases, and 14.3% of stage 3 cases.
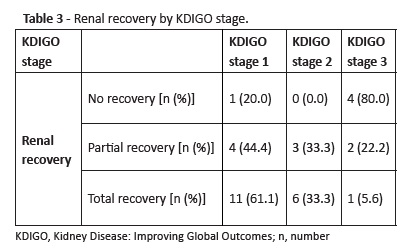
Total renal recovery was reported in 18 cases (56.3%), partial recovery in nine cases (28.1%), and no recovery’ in five cases (15.6%). Urinary output was the only criterion used for diagnosis in 55.5% of cases with partial renal recovery.Distribution of renal recovery by KDIGO stages is described in Table 3.
Renal replacement therapy (RRT) was used in five patients, accounting for 15.6% of AKI cases. All five patients had stage 3 AKI and underwent continuous venovenous hemodiafiltration, with a median duration of four days (interquartile range [IQR]: 1.5-5.5 days). Total renal function recovery was reported for one patient, partial recovery for one patient, and no recovery’ for three patients. Six deaths (5.4%) were reported during the study period, five of which corresponding to AKI cases (one AKI stage 1 and four AKI stage 3). Shock was the cause of death in the five AKI cases, four (80.0%) of which due to multiorgan failure (two cases of septic shock [40.0%] and two cases of cardiogenic shock [40.0%]) and one (20.0%) due to hypovolemic shock.
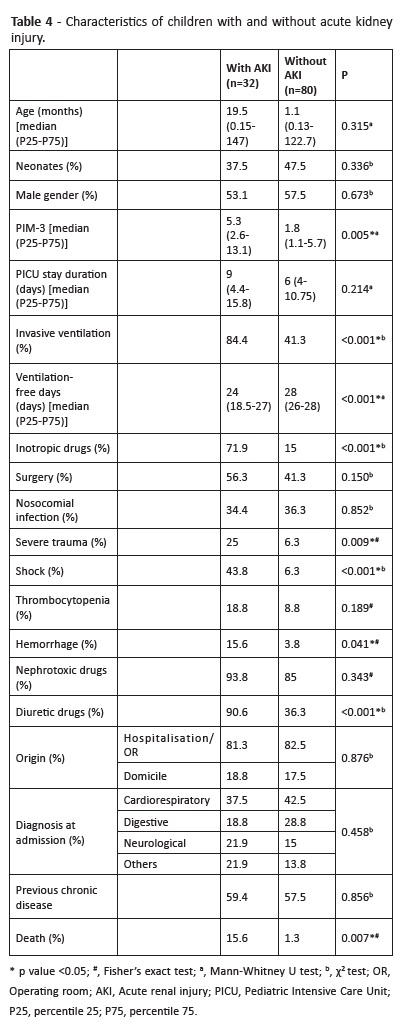
Higher PIM-3 score on admission, severe trauma, shock, hemorrhage, use of inotropic drugs, invasive ventilation, and longer duration of ventilation were significantly more frequent in children with AKI. Additionally, the use of diuretic drugs was also more frequent in children with AKI. The use of nephrotoxic drugs was not associated with a higher incidence of AKI (Table 4).
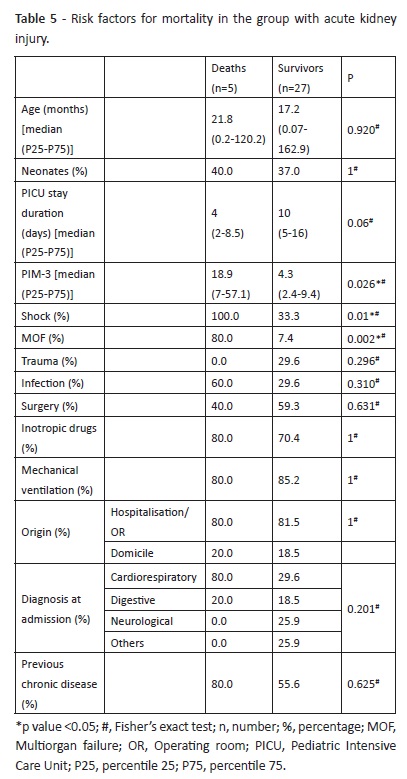
Mortality rate was 15.6% in children with AKI and 1.3% in children without AKI (p=0.007). Shock and multiorgan failure were significantly more frequent in children with AKI who died (p=0.01 and 0.002, respectively) (Table 5).
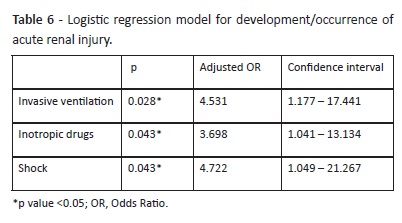
Regression analysis revealed that the presence of shock, use of inotropic drugs, and need for invasive mechanical ventilation were independent risk factors for development of AKI (Table 6). These factors explained the development of AKI in 46.3% of cases. According to the Hosmer and Lemeshow test, the model adequately fitted the data (p> 0.05). PIM-3 and hemorrhage were not included in the model, as they decreased its explanation percentage.
Discussion
The present study confirms the high incidence of AKI in the pediatric intensive care setup and its complexity and multifactorial etiology, which contribute to a significant morbimortality in patients which, due to their clinical condition, are particularly susceptible and require special care.25
AKI was diagnosed in 28.6% of cases admitted to PICU. To date, no similar studies have been developed in Portugal to enable a comparison with characteristics described in the national population. Consequently, only comparisons with international data are possible, which is considerably variable among studies. This variability may be explained by the difficulty in standardizing diagnostic criteria between studies, as well as by heterogeneity in characteristics of different PICUs.4
Considering KDIGO classification and the use of both urinary output and serum creatinine criteria, the incidence of AKI in this study was lower than the 37.4−47.0% incidence reported by others.8,26,27 Nevertheless, the classification criteria adopted is not the only factor influencing AKI diagnosis. The fact that it is based on serum creatinine elevation from baseline and on the reduction of urine output makes the incidence of this condition difficult to estimate in a pediatric population, especially in newborns. Alternative diagnostic criteria adapted to this age group are under investigation.7 Regarding creatinine baseline value, it is not always available in the pediatric population and its calculation depends on the criterion used, resulting in data analysis biases and important discrepancies between results reported by different studies.5,6,28-30 Additionally, some studies do not exclude children with previous kidney disease, with increased susceptibility to acute exacerbation, making comparisons even more challenging.17
In this study, demographic analysis of groups with and without AKI did not reveal significant differences regarding age or gender. This disagrees with other studies reporting a higher incidence of AKI in males and younger children.7,18,31,32 Also no significant differences were found between groups regarding origin, reasons for admission, comorbidities, and PICU length of stay. Similarly to reports in the literature, also in this study they were higher in children with AKI, but with no statistical significance.4, 26
AKI diagnosis was mostly performed until the second day of admission, supporting the notion that AKI complicates hospitalizations mainly until day seven, with most patients diagnosed within the first 72 hours. 6,9,13,25,33-35
Regarding KDIGO stage at diagnosis, 78.1% of cases fulfilled stage 1 criteria and only 21.9% stage 3 criteria, which contrasts with a previous retrospective study conducted at our hospital showing that 80.0% of children fulfilled KDIGO stage 3 at the time of diagnosis.21 As previously mentioned, the high percentage of severe cases observed in the present study may represent a bias associated with retrospective data collection, that preferentially identifies cases codified in clinical records.
Although a direct comparison between different classification systems is not fully accurate, results retrieved highlight the importance of developing prospective studies using AKI criteria in a systematic way, in order to increase sensitivity for AKI identification. Regarding diagnosis, this study reinforces the importance of using both serum creatinine and urine output as criteria for AKI diagnosis, as previously described.8 Although the urinary output criterion used in the three AKI classifications was derived from adult studies to the pediatric population, oliguria may have a significant prognostic impact, making its adaptation relevant, especially for infants.5,26,27 Indeed, the urinary output - which has not been considered in several previous studies due its difficult measurement accuracy − may allow a higher diagnostic sensitivity.13 In this study, this was the only criterion in some KDIGO stage 2 and 3 cases, as well as in a large number of children with only partial renal function recovery. As described in adult studies, oliguria − when stablished − allows AKI diagnosis before an increase in serum creatinine is detected. This usually occurs later or may not even occur, particularly in malnourished children with poor muscle mass, or due to a dilution effect related to volume expansion.8,9 Thus, despite the need for better definitions and adaptations to the heterogeneity of the pediatric population, urinary output should be considered an important AKI diagnostic criterion, besides creatinine, as it may indicate progression to more severe stages of the disease and incomplete renal function recovery. Concerning prognosis at the time of PICU discharge, more than half of patients experienced total recovery. Those were mainly KDIGO stage 1 and 2 patients. Most patients with no recovery were KDIGO stage 3.
Five of the seven KDIGO stage 3 children required RRT, three of which died. More severe AKI cases − corresponding to those with higher KDIGO stage − were associated with increased need for RRT, absence of renal function recovery, and death, as previously reported.4,18 Regarding the association between AKI and increased mortality, Sutherland et al showed that this association remains true despite AKI severity, and also that higher severity is associated with higher mortality rates.4 In the same study, the authors suggested that AKI may present as two relatively distinct disease entities within and outside the pediatric intensive care (where a higher number of factors usually contribute to mortality) and also that less severe AKI grades may be associated with worse outcomes. AKI-associated mortality is mainly due to the fact that these patients are seriously ill, contributing to an increased risk that translates into a higher PIM- 3 score, which seems to be more significant than the unequivocal association with AKI.18,32 This may justify the observed association between hemodynamic shock, MOF, and mortality among children with AKI. Although this entity is not the direct cause of death in this patient population, it may have been a consequence of shock observed in all death cases and a part of MOF simultaneously, thus contributing to aggravate clinical condition and worsen prognosis.
Factors associated with AKI development were consistent with other reports, including independent factors (need for invasive ventilation, use of vasoactive drugs, and shock).5,18,36 As a possible explanation, all these factors are associated with worse clinical condition and require more therapeutics and support techniques, increasing AKI risk.
Invasive ventilation is a previously described risk factor and AKI is also associated with longer ventilation time demands.5,6 explain this, it has been suggested that ventilation poses an increased risk for variations on selective renal vasoconstriction hemodynamics due to sympathetic stimulation.37 In this study, ventilation was independently associated with a 4.5-fold higher risk of AKI and, although ventilation-free-days were not an independent risk factor, a significantly lower number of days without ventilation was observed in the AKI group.
The relationship between vasoactive support and AKI, while well documented, remains a subject of debate. Several studies reported the use of inotropic drugs as a risk factor for AKI, similarly to results here presented.32,35 These drugs are generally used for treatment of systemic hypotension refractory to fluid therapy, often in the context of shock, hemorrhage, or MOF. However, they also act on the renal vasculature, with its mechanism not fully understood yet.38,39 Hemodynamic instability, which leads to inotropic drug use, is associated with renal hypoperfusion, resulting in renal damage of prerenal cause, and making it difficult to understand the role of inotropic drugs in AKI.35
The association between AKI and the use of diuretic drugs requires caution, since these drugs are often initially used to evaluate diuretic response in oliguric patients. Although diuretics are potentially nephrotoxic, they are often necessary to improve the child’s urinary output, making it difficult to clarify their effect as a therapeutic or pernicious agent.11 The absence of association of AKI with other nephrotoxic drugs, although surprising, may be attributed to the careful evaluation required to use these agents (including their dose adjustments) in a safe manner.17
Increasing evidence is emerging about the benefits of using earlier and more sensitive biomarkers for the diagnosis of AKI. Since elevation of serum creatinine values depends on the child’s age and muscle mass, this marker may overestimate renal function, on the one hand, or be modified by the child’s clinical condition, on the other. Furthermore, it is acknowledged that an abrupt decrease in serum creatinine may occur in cases of renal dysfunction − due to increased tubular secretion − to compensate glomerular filtration rate reduction, hampering diagnosis with currently used criteria.28 Overall, new molecules begin to replace the classic markers of AKI diagnosis. Both cystatin C and NGAL anticipate the diagnosis of AKI in 24 to 72 hours, and this difference may be key for timely therapeutic intervention.28 more studies are needed before these markers enter clinical practice.27
The present study has some limitations that can be circumvented in future studies, including its reduced sample size. A large multicenter study, including several pediatric intensive care units, will potentially enable a better disease characterization and confirm results here obtained. On the other hand, a more accurate and detailed registration of some variables of interest - including detailed drug dosage, inotropic drug support, and invasive ventilation − may improve the discriminatory strength of identified risk factors and clarify their temporal relation with AKI development. Regardless of these limitations, to the best of our knowledge this is the first Portuguese study on AKI in the pediatric intensive care setting with prospective data collection and may be a preliminary analysis for future research in the area.
Conclusion
AKI is a common complication in PICU. It is observed in approximately one quarter of admissions, occurring mostly until its second day.
The use of validated AKI definitions may improve diagnostic sensitivity, with both serum creatinine and urinary output criteria being relevant for early diagnosis.
Some factors have been identify which may contribute to AKI development during PICU stay, such as shock and need for invasive mechanical ventilation and vasoactive drugs. When identified, clinical suspicion and a careful monitoring of renal function should be granted. Shock was a risk factor for mortality in this group of children. Identification of risk factors is important for careful clinical monitoring and key for an early diagnosis and treatment, allowing a better prognosis. However, large prospective multicentric studies including new biomarkers of renal injury are required.
REFERENCES
1. Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Behrman RE. Nelson Textbook of Pediatrics. 20th ed. 2016. p. 2539-43. [ Links ]
2. Nichols DG, Shaffner DH. Rogers’ Textbook of Pediatric Intensive Care. 2015.
3. Keenswijk W, Vanmassenhove J, Raes A, Dhont E, VandeWalle J. Epidemiology and outcome of acute kidney injury in children, a single center study. Acta Clin Belg. 2017; 3286:1-8. [ Links ]
4. Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015; 10:554-61. [ Links ]
5. Miklaszewska M, Korohoda P, Sobczak A, Horbaczewska A, Filipiak A, Zachwieja K, et al. Acute kidney injury in a single pediatric intensive care unit in poland: A retrospective study. Kidney Blood Press Res. 2014; 39:28-39. [ Links ]
6. Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011; 15:R146. [ Links ]
7. Marin T, Derossett B, Bhatia J. Urinary Biomarkers to Predict Neonatal Acute Kidney Injury. J Perinat Neonat Nurs. 2018; 0:1-9. [ Links ]
8. Koeze J, Keus F, Dieperink W, Van der Horst ICC, Zijlstra JG, Van Meurs M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017; 18:1-9. [ Links ]
9. Nelson Sanchez-Pinto L, Goldstein SL, Schneider JB, Khemani RG. Association between progression and improvement of acute kidney injury and mortality in critically ill children. Pediatr Crit Care Med. 2015; 16:703-10. [ Links ]
10. Glanzmann C, Frey B, Vonbach P, Meier CR. Drugs as risk factors of acute kidney injury in critically ill children. Pediatr Nephrol. 2016; 31:145-51. [ Links ]
11. Bonazza S, Bresee LC, Kraft T, Ross BC, Dersch-Mills D. Frequency of and Risk Factors for Acute Kidney Injury Associated With Vancomycin Use in the Pediatric Intensive Care Unit. J Pediatr Pharmacol Ther. 2016; 21:486-93. [ Links ]
12. Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010; 38:933-9. [ Links ]
13. Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. 2017; 376:11-20. [ Links ]
14. American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005; 16:1886-903. [ Links ]
15. Mussap M, Noto A, Fanos V, Van Den Anker JN. Emerging biomarkers and metabolomics for assessing toxic nephropathy and acute kidney injury (AKI) in neonatology. Biomed Res Int. 2014; 602526 [ Links ]
16. Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008; 73:1008-16. [ Links ]
17. Serna-Higuita LM, Nieto-Ríos JF, Contreras-Saldarriaga JE, Escobar-Cataño JF, Gómez-Ramírez LA, Montoya-Giraldo JD, et al. Risk factors for acute kidney injury in a pediatric intensive care unit: a retrospective cohort study. Medwave. 2017; 17:e6940. [ Links ]
18. Gupta S, Sengar GS, Meti PK, Lahoti A, Beniwal M, Kumawat M. Acute kidney injury in Pediatric Intensive Care Unit: Incidence, risk factors, and outcome. Indian J Crit Care Med. 2016; 20:526- 9. [ Links ]
19. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012; 2:1-138. [ Links ]
20. Ceriotti F, Boyd JC, Klein G, Henny J, Queraltó J, Kairisto V, et al. Reference intervals for serum creatinine concentrations: Assessment of available data for global application. Clin Chem. 2008; 54:559-66. [ Links ]
21. Mendes da Silva C, do Carmo C, Pinto C, Januário G. Acute kidney injury in children at a Portuguese tertiary care center. [ Links ]
22. Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator- free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002; 30:1772-7. [ Links ]
23. Straney L, Clements A, Parslow RC, Pearson G, Shann F, Alexander J, et al. Paediatric index of mortality 3: An updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med. 2013; 14:673-81. [ Links ]
24. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004; 114:555-76. [ Links ]
25. Basu RK, Devarajan P, Wong H, Wheeler DS. An update and review of acute kidney injury in pediatrics. Pediatr Crit Care Med. 2011; 12:339-47. [ Links ]
26. Kari JA, Alhasan KA, Shalaby MA, Khathlan N, Safdar OY, Al Rezgan SA, et al. Outcome of pediatric acute kidney injury: a multicenter prospective cohort study. Pediatr Nephrol. 2018; 33:335-40. DOI:10.1007/s00467-017-3786-1. Epub 2017. [ Links ]
27. Palermo J, Dart AB, De Mello A, Devarajan P, Gottesman R, Garcia Guerra G, et al. Biomarkers for Early Acute Kidney Injury Diagnosis and Severity Prediction: A Pilot Multicenter Canadian Study of Children Admitted to the ICU. Pediatr Crit Care Med. 2017; 18:e235-44. [ Links ]
28. Zappitelli M. Epidemiology and Diagnosis of Acute Kidney Injury. Semin Nephrol. 2008; 28:436-46. [ Links ]
29. Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008; 3:948-54. [ Links ]
30. Hessey E, Ali R, Dorais M, Morissette G, Pizzi M, Rink N, et al. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr Nephrol. 2017; 32:1953-62. [ Links ]
31. Ghobrial EE, Elhouchi SZ, Eltatawy SS, Beshara LO. Risk factors associated with acute kidney injury in extremely. Vol. 29. 2018. p. 81-7. [ Links ]
32. Mehta P, Sinha A, Sami A, Hari P, Kalaivani M, Gulati A, et al. Incidence of acute kidney injury in hospitalized children. Indian Pediatr. 2012; 49:537-42. [ Links ]
33. Sanchez-Pinto LN, Khemani RG. Development of a Prediction Model of Early Acute Kidney Injury in Critically Ill Children Using Electronic Health Record Data. Pediatr Crit Care Med. 2016; 17:508-15. [ Links ]
34. Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007; 71:1028-35. [ Links ]
35. Soler YA, Nives-Plaza M, Prieto M, Jesús RG-D, Suárez-Riviera M. pRIFLE (Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease) score identifies Acute Kidney Injury and predicts mortality in critically ill children: a prospective study. Pediatr Crit Care Med. 2013; 14:e189-95. [ Links ]
36. Krishnamurthy S, Narayanan P, Prabha S, Mondal N, Mahadevan S, Srinivasan S, et al. Clinical profile of acute kidney injury in a pediatric intensive care unit from Southern India: A prospective observational study. Indian J Crit Care Med. 2013; 17:207-13. [ Links ]
37. van den Akker JPC, Egal M, Groeneveld JAB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013; 17:R98-39. [ Links ]
38. Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med. 2008; 36:179-86. [ Links ]
39. Lauschke A, Teichgräber UKM, Frei U, Eckardt K-U. “Low-dose” dopamine worsens renal perfusion in patients with acute renal failure. Kidney Int. 2006; 69:1669-74.
40. Haines RW, Lin S-P, Hewson R, Kirwan CJ, Torrance HD, O’Dwyer MJ, et al. Acute Kidney Injury in Trauma Patients Admitted to Critical Care: Development and Validation of a Diagnostic Prediction Model. Sci Rep. 2018; 8:3665.
Endereço para correspondência | Dirección para correspondencia | Correspondence
João Rio Martins
Pediatric Intensive Care Unit
Hospital Pediátrico de Coimbra
Centro Hospitalar e Universitário de Coimbra
Av. Afonso Romão
3000-602 Coimbra
Email: joaoriomartins@gmail.com
Received for publication: 01.08.2018
Accepted in revised form: 25.01.2019