Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Nascer e Crescer
versão impressa ISSN 0872-0754versão On-line ISSN 2183-9417
Nascer e Crescer vol.28 no.3 Porto set. 2019
https://doi.org/10.25753/BirthGrowthMJ.v28.i3.15671
REVIEW ARTICLES | ARTIGOS DE REVISÃO
Association between food allergy and otitis media with effusion in light of current knowledge
Associação entre alergia alimentar e otite média serosa à luz do conhecimento atual
Gonçalo Jorge MendesI, Lise BrosseronII, Inês LopesII, António MagalhãesI, Cecília Almeida e SousaI
I - Department of Otorhinolaryngology and Cervicofacial Surgery, Centro Hospitalar e Universitário do Porto. 4099-001 Porto, Portugal. gonmendes@hotmail.com; almeidascecilia@gmail.com; aatm@netcabo.pt
II - Department Immunoallergology, Centro Hospitalar de Vila Nova de Gaia/Espinho. 4434-502 Vila Nova de Gaia, Portugal. lisesbb@gmail.com; inesmarqueslopes@sapo.pt
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Introduction: Otitis media with effusion (OME) is a common middle ear condition in childhood with possible short- and long-term complications regarding hearing, language, cognition, and inflammatory disorders. Food allergy (FA) is also frequent in children and has a seemingly growing prevalence. It can affect several organs, including the middle ear.
Objectives: To review published literature regarding the potential association of these conditions and their relative risk.
Literature review: Allergy is a factor commonly associated with increased OME risk. Most literature on the topic focuses allergic rhinitis, due to local mechanisms potentially implicated in middle ear pathology. FA mostly affects young children, with incidence peaking at one year of age, and seems to be linked to a higher risk of other future atopic manifestations, including allergic rhinitis and asthma. OME has been proposed as an atopic manifestation in the middle ear, with several authors developing clinical studies on the subject since 1958 until present. Results have been occasionally conflicting, both regarding relative prevalence rates and presence or absence of a significant correlation between OME and FA.
Conclusions: Despite absence of consensus, OME and FA frequencies, as well as their potentially severe effects and often refractory course, highlight the need for clinicians to be alert to their potential association. Further clinical studies are required to clarify this important topic.
Keywords: food allergy; hearing loss; otitis media with effusion
RESUMO
Introdução: A otite média serosa (OMS) é uma patologia frequente do ouvido médio na infância, com possíveis efeitos adversos a curto e longo prazo na audição, linguagem, cognição e alterações inflamatórias. A alergia alimentar também é frequente em crianças, com prevalência crescente, podendo implicar o atingimento de vários órgãos, nomeadamente o ouvido médio.
Objectivos: Efetuar uma revisão da literatura publicada acerca da possível associação entre ambas as patologias e respetivo risco relativo.
Desenvolvimento: A alergia é um dos fatores de risco apontados para o desenvolvimento de OMS. A maior parte da literatura foca sobretudo a rinite alérgica, dado o possível contributo de mecanismos locais para a patologia do ouvido médio. A alergia alimentar afeta sobretudo crianças pequenas, com um pico de incidência por volta do ano de idade. Parece implicar também um maior risco de outras manifestações atópicas no futuro, como rinite alérgica ou asma. A OMS foi proposta como possível manifestação atópica no ouvido médio, tendo vários autores desenvolvido estudos clínicos sobre o tema desde 1958 até ao presente. Os resultados obtidos foram por vezes díspares, nomeadamente em termos de prevalência relativa e presença ou ausência de uma correlação significativa entre ambas as condições.
Conclusões: Apesar da falta de consenso, a frequência de OMS e de alergia alimentar, suas possíveis complicações graves e evolução clínica por vezes lenta, obrigam a um grau de suspeição elevado em relação a uma possível associação entre ambas. São necessários mais estudos que permitam clarificar este tema relevante.
Palavras-chave: alergia alimentar; perda de audição; otite média serosa
Introduction
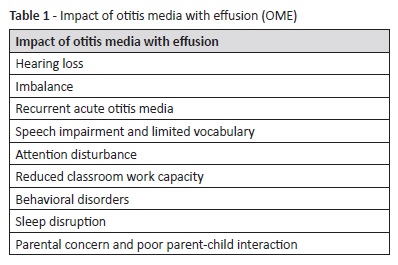
Otitis media with effusion (OME), or serous otitis media, is defined as a fluid collection in the middle ear without acute signs of infection. It is the most common hearing loss etiology during childhood, being also common in asymptomatic young children.1 The condition is highly relevant because, in addition to inflammatory and structural ear pathologies, as recurrent acute otitis media (AOM) and tympanic membrane atrophy or atelectasis, it may be responsible for hearing loss during children’s critical process of language development (Table 1).2 Therefore, mechanisms leading to OME development should be recognized to enable preventive and treatment measures. OME seems to be a multifactorial disease with several important risk factors, such as family history of otitis media, bottle feeding, day-care centre attendance, adenoidal hypertrophy, Eustachian tube dysfunction, craniofacial abnormalities, exposure to tobacco smoke, and low socioeconomic status.3 Allergy has also been proposed as an etiological factor. Respiratory allergic manifestations, as allergic rhinitis, have been the most extensively studied, but the association between food allergy and OME also seems relevant and has been the object of clinical investigation.1 Nevertheless, some reports state that allergy is not more common in OME children than in the normal pediatric population.4 Food allergy is an important cause of pediatric morbidity.5 It is defined as an immunological reaction against alimentary products that may affect any organ in the body, including the middle ear. Manifestations can be cutaneous, cardiovascular, or involving the aerodigestive tract.
Objectives
The aim of this study was to review current literature regarding the association of OME and food allergy and respective relative risks.
Literature review
OME is a common disorder, with an estimated prevalence of 10−17% from two to four years and 3−4% from six to eight years. By the age of four, 90% of children are estimated to have suffered from OME at least once.6 In the United States of America, approximately 2.2 million episodes of OME are diagnosed every year, with high direct and indirect costs.7 In European countries, studies have documented prevalence rates between 8.7% and 42%, with a reported prevalence of 9.7% in Portugal.8 Prevalence in children with craniofacial abnormalities is considerably higher, ranging between 60% and 85%.9 OME may arise spontaneously, during upper respiratory infections, or following an episode of acute otitis media.10 In most cases, OME will resolve within three months or less without treatment, but at least 25% of cases will persist after this period and 5−10% will persist after one year.11 Furthermore, 30−40% of children are estimated to suffer from repeated OME episodes.11 OME can result in hearing loss, imbalance, low school productivity, behavioral changes, discomfort, and recurrent AOM.12 Additionally, it also has negative implications on caregivers’ well-being and in patient-caregiver interaction.13
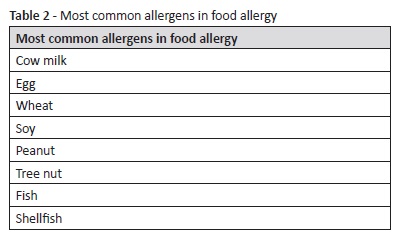
Food allergy is a type of adverse food reaction which refers to immunologically mediated reactions. It is a consequence of an abnormal immunologic reaction following ingestion of a specific alimentary product.14 Prevalence of food allergy or sensitization is calculated to vary between 5% and 10% in young children, peaking around the age of one and progressively declining until late childhood.15 The global prevalence of food allergy in children appears to be increasing over the last years.16,17 Among the most commonly involved food items is cow milk, egg, wheat, soy, peanut, tree nut, fish, and shellfish (Table 2).18 Symptoms and signs can involve acute urticaria and angioedema, oropharyngeal pruritus and swelling, asthma, nasal and conjunctival irritation, nausea, abdominal pain, cramping, vomiting, or diarrhea. In more severe reactions, anaphylaxis can pose a vital risk. Different criteria have been used in different studies to evaluate food allergy. If sensitization is assessed − through skin testing or serum-specific IgE dosing − higher prevalence rates are obtained compared with clinical reactivity analysis. This occurs because some patients with detectable specific IgE antibodies have no symptoms upon food ingestion, while only rarely will patients with documented clinical reactions to specific items have low levels of specific IgE upon testing.19 Children with food allergy or sensitization have a higher probability of subsequently developing allergic rhinitis and asthma.20
Both OME and food allergy have an underlying inflammatory mechanism. The middle ear is considered to be a modified physiologic aerated cavity, in which pressure is maintained at atmospheric level through mucosal gas exchange and Eustachian tube ventilation.21 The pathogenesis of OME appears to be complex and have an inflammatory nature, and is characterized by excessive mucus production, mucosal hyperplasia, and Eustachian tube function impairment. Most often, this process is thought to be secondary to bacterial activity, although it has also been correlated with other factors, such as genetic predisposition, gastroesophageal reflux, and allergy.22 The role of allergy in the etiology of OME is, however, a matter of controversy. Several studies on the subject have been conducted since 1929, mostly regarding the influence of inhalant allergy and allergic rhinitis.23,24 In fact, OME is often a comorbidity of allergic rhinitis, while having an apparently lower risk in non-allergic rhinitis.25 This association has been interpreted as resulting from inflammatory obstruction of the nose, presence of bacteria-populated allergic secretions in Eustachian tube proximity, or inflammatory swelling of the Eustachian tube mucosa, particularly near its nasopharyngeal extremity.25
Subsequent studies hypothesized that the middle ear itself can be a target organ of allergic disease, and that other mechanisms are relevant in OME pathogenesis besides local and mechanical processes only.26 Allergic mediators such as IgE, activated mast cells, tryptase, or myeloperoxidase have been detected and analyzed in the middle ear of patients with OME.27 Other studies investigated, not only middle ear effusion, but also mucosal levels of the same allergic inflammation markers, also considering additional parameters as eosinophil counts, Th2-type cytokines, and their messenger RNA.28 Studies in sensitized animals showed that a specific antigenic challenge to the middle ear mucosa induced inflammation compatible with a direct allergic reaction.29 On the other hand, small changes were observed in animal middle ear mucosa when allergens were introduced in the nose and pharynx, suggesting that allergic changes in middle ear mucosa cannot be fully explained by direct allergen spread via the Eustachian tube. While allergic rhinitis and nasopharyngitis may not suffice to induce OME, a longer disease duration seems apparent when they are present.21 The allergic process may also induce delivery of inflammatory mediators to middle ear microcirculation, besides local changes in blood flow and gas exchange. It has hence been suggested that immune complexes involving food allergens, particularly with dairy products, may play a relevant role in promoting OME, and that it can be a unique mechanism for food allergy-elicited OME.24
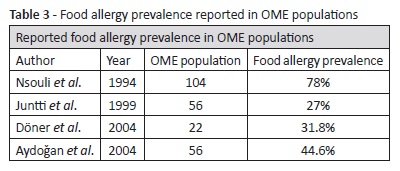
Despite such pathophysiological research and the theoretical association between OME and food allergy, literature reporting clinical investigation data is not as comprehensive as expected. The association was firstly described in 1958 in a report of 50 OME children treated for allergy, stressing the importance of food allergy in allergic management.30 In a 1961 study, 50% of 82 OME patients evidenced food allergy and more than 80% improved with allergy treatment (inhalant allergen immunotherapy combined with elimination of food allergens).31 Although not reporting the relative prevalence rates of OME and food allergy, another study described OME improvement with elimination diet.32 Other authors advocate the role of elimination diets in OME treatment, when in association with food allergy.4 A widely cited article by Nsouli et al. described one of the highest food allergy prevalence rates in the literature in a group of 104 OME children: approximately 78%.33 These children were randomly selected between OME patients and evaluated for food allergy through skin prick testing, specific IgE testing, and food challenge. The authors also showed a significant OME improvement with elimination diet and OME recurrence after subsequent challenge. Another study from 1994 reported that food allergy, particularly to cow milk, egg white, and soybean, was more prevalent in OME children than in controls and that it may account for a significant percentage of OME cases.34 However, this study stressed the controversy around the topic and also quoted some studies with disparate results.34-37
Derebery et al. investigated 151 patients with Eustachian tube dysfunction and inhalant allergy, of which 92.3% had clinical food allergy. Patients showed significant improvement with allergy treatment after failure of other OME treatment options, with adherence to elimination diets (when indicated) proving to be significantly related to outcomes.38 In a Finnish study focusing cow milk allergy, a higher risk of recurrent AOM but not of OME was shown, although patients with any atopic manifestations did show a higher prevalence of OME globally.39 A 2004 study directly focusing the role of food allergy in OME showed that 44.6% of a group of 25 OME patients had food allergy (as opposed to 18% in the control group), while OME was identified in 25% of 28 patients followed for food allergy (3% in the control group), which proved to be statistically significant.1 Doner et al. analyzed a group of 22 patients with recurrent OME after adenoidectomy and ventilation tube placement, of which 31.8% were positive for food allergy, most frequently to egg white, back wheat, and plum (Table 3).2
Conclusions
OME and food allergy are common disorders that may significantly impact patients’ development and well-being. Incidence of food allergy, in particular, appears to be rising in recent years. Both OME and food allergy are multifactorial conditions with complex pathogenesis, not fully clear to date. Their association and the contribution of food allergy to OME remain controversial, and a consensus about their relative prevalence has not yet been reached. Although some authors disagree with the association between OME and food allergy, the body of evidence regarding their mutual relevance is slowly increasing. Nevertheless, more clinical studies and larger patient series are clearly necessary to address this subject and clarify the association between OME and food allergy. In light of current knowledge, it is wise for clinicians managing OME to be alert to the possibility of an underlying food allergy, particularly in patients with conditions refractory to initial therapeutic measures or with cutaneous, cardiovascular, or aerodigestive tract symptoms compatible with food allergy. Egg white, cow milk, soybean, back wheat, and plum are some of the most likely agents potentially implicated in food allergy cases, and should be promptly identified and treated to improve middle ear status and prevent food allergy complications. Similarly, doctors caring for children with food allergy should keep in mind that concomitant OME may be necessary to exclude, in order to prevent its short- and long-term negative effects.
Highlights
· Otitis media with effusion and food allergy are common disorders, with increasing prevalence of the latter.
· Presence of both conditions combined can have severe and long-term negative effects in patients’ health, namely regarding hearing, language, cognitive development, and atopic manifestations.
· The relationship between otitis media with effusion and food allergy is still controversial, although the body of evidence is slowly increasing.
· Clinicians managing patients with these conditions should be alert to their possible association and exclude it upon suspicion.
· Further studies and larger series are required to clarify this controversial topic.
REFERENCES
1. Aydoğan B, Kiroğlu M, Altintas D, Yilmaz M, Yorgancilar E, Tuncer U. The role of food allergy in otitis media with effusion. Otolaryngol Head Neck Surg. 2004; 130:747-50. [ Links ]
2. Dӧner F, Yariktas M, Demirci M. The role of allergy in recurrent otitis media with effusion. J Investig Allergol Clin Immunol. 2004; 14:154-8. [ Links ]
3. Williamson I. Otitis media with effusion in children. BMJ Clin Evid. 2011; 2011. pii: 0502. [ Links ]
4. Ruokonen J, Paganus A, Lehti H. Elimination diets in the treatment of secretory otitis media. International journal of pediatric otorhinolaryngology. 1982; 4:39-46. [ Links ]
5. Haywood M, Alade A, Vijendren A, Singh P. Late presentation of egg white and milk protein allergy as rhinitis and otitis media. British journal of hospital medicine. 2017; 78:112-3. [ Links ]
6. Tos M. Epidemiology and natural history of secretory otitis. Otology & Neurotology. 1984; 5: 459-62. [ Links ]
7. Shekelle P, Takata G, Chan LS, Mangione-Smith R, Corley PM, Morphew T, et al. Diagnosis, natural history, and late effects of otitis media with effusion. Evid Rep Technol Assess (Summ). 2002; 55:1-5. [ Links ]
8. Correia A. O desenvolvimento fonológico de crianças com otites médias com derrame: estudo longitudinal. 2015. [ Links ]
9. Maris M, Wojciechowski M, Van de Heyning P, Boudewyns A. A cross-sectional analysis of otitis media with effusion in children with Down syndrome. Eur J Pediatr. 2014; 173:1319-25. [ Links ]
10. Paradise JL, Rockette HE, Colborn DK, Bernard BS, Smith CG, Kurs-Lasky M, et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997; 99:318-33. [ Links ]
11. Williamson IG, Dunleavey J, Bain J, Robinson D. The natural history of otitis media with effusion-a three-year study of the incidence and prevalence of abnormal tympanograms in four South West Hampshire infant and first schools. J Laryngol Otol. 1994; 108:930-4. [ Links ]
12. Rosenfeld RM, Schwartz SR, Pynnonen MA, Tunkel DE, Hussey HM, Fichera JS, et al. Clinical practice guideline: tympanostomy tubes in children. Otolaryngol Head Neck Surg. 2013; 149:S1- 35. [ Links ]
13. Brower CNM, Maille AR, Rovers MM , Grobbee DE, Sanders EAM, Schilder AGM. Health-related quality of life in children with otitis media. International journal of pediatric otorhinolaryngology. 2005; 69:1031-41. [ Links ]
14. Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014; 134:1016-25.e43. [ Links ]
15. Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011; 127:668-76.e1-2 [ Links ]
16. Jackson KD, Howie LD, Akinbami OJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief. 2013:1-8. [ Links ]
17. Woicka-Kolejwa K, Zaczeniuk M, Majak P, Pawłowska- Iwanicka K, Kopka M, Stelmach W, et al. Food allergy is associated with recurrent respiratory tract infections during childhood. Advances in Dermatology and Allergology/Postȩpy Dermatologii i Alergologii. 2016; 33:109-13. [ Links ]
18. Allen JK, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin N Am. 2012; 32:35-50. [ Links ]
19. Wood RA. Food allergy in children: prevalence, natural history and monitoring for resolution. UpToDate online. Accessed Oct, 2018. [ Links ]
20. Sicherer SH. Respiratory manifestations of food allergy. UpToDate online. Accessed Oct, 2018. [ Links ]
21. Bernstein JM. Role of allergy in eustachian tube blockage and otitis media with effusion: a review. Otolaryngology-head and neck surgery. 1996; 114: 562-8. [ Links ]
22. Marseglia G, Pagella F, Caimmi D, Caimmi S, Castellazzi AM, Poddighe D, et al. Increased risk of otitis media with effusion in allergic children presenting with adenoiditis. Otolaryngology - Head and Neck Surgery. 2008; 138: 572-5. [ Links ]
23. Luong A, Roland PS. The link between allergic rhinitis and chronic otitis media with effusion in atopic patients. Otolaryngologic Clinics of North America. 2008; 41: 311-23. [ Links ]
24. Bernstein JM. The role of IgE-mediated hypersensitivity in the development of otitis media with effusion. Otolaryngologic Clinics of North America. 1992; 25:197-211. [ Links ]
25. Zermotti M, Pawankar R, Ansotegui I, Badellino H, Croce JS, Hossny E, et al. Otitis media with effusion and atopy: is there a causal relationship?. World Allergy Organization Journal. 2017; 10:37. [ Links ]
26. Ramakrishnan JB. The role of food allergy in otolaryngology disorders. Current opinion in otolaryngology & head and neck surgery. 2010; 18:195-9. [ Links ]
27. Hurst DS, Venge P. The impact of atopy on neutrophil activity in middle ear effusion from children and adults with chronic otitis media. Archives of Otolaryngology-Head & Neck Surgery. 2002; 128:561-6. [ Links ]
28. Doyle WJ. The link between allergic rhinitis and otitis media. Current opinion in allergy and clinical immunology. 2002; 2:21- 5. [ Links ]
29. Yamashita T, Okazaki N, Kumazawa T. Relation between Nasal and Middle Ear Allergy Experimental Study. Annals of Otology, Rhinology & Laryngology. 1980; 89: 147-52. [ Links ]
30. Solow IA. Is serous otitis media due to allergy or infection. Annals of allergy. 1958; 16:297. [ Links ]
31. Lecks HI. Allergic aspects of serous otitis media in childhood. New York state journal of medicine. 1961; 61:2737. [ Links ]
32. Viscomi GJ. Allergic secretory otitis media: an approach to management. The Laryngoscope. 1975; 85:751-8. [ Links ]
33. Nsouli TM, Nsouli SM, Linde RE, O’Mara F, Scanlon RT, Bellanti JA. Role of food allergy in serous otitis media. Annals of allergy. 1994; 73:215-9.
34. Corey JP, Adham RE, Abbass AH, Seligman I. The role of IgE-mediated hypersensitivity in otitis media with effusion. American journal of otolaryngology. 1994; 15:138-44. [ Links ]
35. Reisman RE, Bernstein J. Allergy and secretory otitis media: clinical and immunologic studies. Pediatric Clinics of North America. 1975; 22:251-7. [ Links ]
36. Bluestone CD. Eustachian tube function and allergy in otitis media. Pediatrics. 1978; 61: 753-60. [ Links ]
37. McGovern JP, Haywood TJ, Fernandez A. Allergy and secretory otitis media: an analysis of 512 cases. JAMA. 1967; 200:124-8. [ Links ]
38. Derebery MJ, Berliner KI. Allergic eustachian tube dysfunction: diagnosis and treatment. The American journal of otology. 1997; 18:160-5. [ Links ]
39. Juntti H, Tikkanen S, Kokkonen J, Alho OP, Niinimäki A. Cow’s milk allergy is associated with recurrent otitis media during childhood. Acta Otolaryngol. 1999; 119:867-73.
Endereço para correspondência | Dirección para correspondencia | Correspondence
Gonçalo Jorge Mendes
Department of Otorhinolaryngology and Cervicofacial Surgery
Centro Hospitalar e Universitário do Porto
Largo Prof. Abel Salazar
4099-001 Porto
Email: gonmendes@hotmail.com
Received for publication: 21.11.2018
Accepted in revised form: 28.03.2019