Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Nascer e Crescer
versão impressa ISSN 0872-0754versão On-line ISSN 2183-9417
Nascer e Crescer vol.28 no.3 Porto set. 2019
https://doi.org/10.25753/BirthGrowthMJ.v28.i3.15730
CASE REPORTS | CASOS CLÍNICOS
Pollen-food syndrome in the adolescent
Síndrome pólen-frutos na adolescência
Joana PitaI, Maria Luís MarquesII, Moisés Labrador-HorrilloIII, Helena FalcãoII, Leonor CunhaII
I - Department of Allergy and Clinical Immunology. Hospital Dona Estefânia. Centro Hospitalar Universitário de Lisboa Central. Lisboa. 1169-045. Portugal. joana.s.pita@gmail.com
II - Department of Allergy and Clinical Immunology. Centro Hospitalar Universitário do Porto. 4099-001 Porto, Portugal. maluis234@gmail.com; falcao.helena@gmail.com; leonorcunhagraca@sapo.pt
III - Allergy Section. Hospital Universitario Vall d’Hebron. 08035 Barcelona, Spain. mlabrador@vhebron.net
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Pollen-food syndrome (PFS) is characterized by allergic symptoms elicited by the ingestion of raw fruits or vegetables in patients with seasonal allergic rhinitis/rhinoconjunctivitis. The estimated prevalence of PFS in patients with pollen allergy is 50-70%. Typically, PFS-induced symptoms are restricted to the oral cavity (oral allergy syndrome - OAS). The authors present the case of a female adolescent with grass pollen allergic rhinitis treated with oral immunotherapy for four years and four months, who developed OAS to fresh fruits and walnuts. Diagnostic workup showed sensitization to several allergens, particularly to profilin, which seems to be responsible for PFS. The patient remains asymptomatic with fresh fruits and walnut avoidance, currently tolerating cooked apple, without symptoms.
Keywords: allergy; pollen-food-syndrome; profilin
RESUMO
O síndrome pólen-frutos (SPF) caracteriza-se por sintomas alérgicos causados pela ingestão de frutas ou vegetais crus em doentes com rinite/ rinoconjuntivite alérgica sazonal. A prevalência estimada de SPF em doentes com polinose é de 50−70%. Tipicamente, o SPF manifesta-se através de sintomas alérgicos localizados na cavidade oral (síndrome de alergia oral − SAO). Os autores descrevem o caso de uma adolescente com rinite alérgica a gramíneas, submetida a imunoterapia oral durante quatro anos e quatro meses, que desenvolveu SAO a frutos frescos e nozes. Os exames complementares de diagnóstico revelaram sensibilização a vários alergénios, particularmente a profilina, aparentemente responsável pelo SPF. A doente permanece assintomática com evicção de todos os frutos frescos e nozes, atualmente tolerando maçã cozinhada, sem sintomas.
Palavras-chave: alergia; síndrome-pólen-frutos; profilina
Background
Seasonal allergic rhinitis (SAR) is common in Europe, with an estimated prevalence of 40%.1 The incidence of seasonal allergic rhinitis is 5% at 4 years old, 8.5% at 6−7 years old, and 19% in pre-adolescents. In 1942, it was reported that some patients developed allergic symptoms after eating certain plant foods.1 The phenomenon occurs when plant molecules cross-react with their homologues in the offending foods (incomplete food allergens, class 2 food allergy).1,2 Pollen-food syndrome (PFS) is characterized by allergic symptoms elicited by fruit or vegetable ingestion in patients with seasonal allergic rhinitis/rhinoconjunctivitis.2 PFS estimated prevalence in patients with pollen allergy is 50-70%.3
PFS is an IgE-mediated entity, with sensitizing reactions occurring between IgE antibodies and cross-reactive pollen allergens. IgE originally generated in response to pollen exposure will also bind to food proteins, producing typical symptoms. The genesis of this immunological phenomenon is not completely understood.1 PFS diagnosis is accomplished through detailed clinical history. Diagnosis may be challenging, with difficulties in differentiating between primary sensitization and immunological cross-reactivity. The relationship between symptoms and sensitization may not be uniform.1 Typical PFS symptoms are restricted to the oral cavity and include immediate oral itching, angioedema of lips, tongue, palate or oropharynx, and laryngeal tightness, as well as paresthesia of these structures. Altogether, this group of symptoms is labeled as oral allergy syndrome (OAS).2,3
Molecules causing PFS are usually labile, degraded by heat and digestive enzymes, and can induce allergic reactions only in already-sensitized patients.2 Primary allergens causing PFS in patients with birch sensitivity are Bet v 1 cross-reactive antigens and profilins. PFS is also commonly observed in individuals sensitized to grass and weed pollen.3
The first described allergenic profilin − Bet v 2 from birch pollen − was identified in 1991, and since then many allergenic profilins have been identified in pollen, plant foods, and latex, indicating a high degree of cross-reactivity due to their common epitopes.4 Profilins are a family of highly conserved proteins, which play a major role in regulating microfilament system activity and intracellular calcium levels. They are essential for cellular activities, and once ubiquitously spread can be designated as “panallergens”, accounting for a large number of allergic sensitizations clearly related to cross-reactivity and co-sensitization between inhalant, latex, and plant-derived food allergens.5 Profilin is considered a minor respiratory allergen.6,7 Concerning its ability to induce food allergy reactions, profilin is accepted as a mild or incomplete food allergen due to its reduced enzymatic and thermal stability, only able to induce local symptoms, such as OAS.8,9 Profilin food allergy is therefore considered a secondary effector of a primary respiratory allergic disease.10 Sensitization to profilin is quite frequent at preschool age (>15%), with an increasing rate with age and disease duration. It is linked to multiple sensitizations to genuine pollen allergenic molecules and is associated with OAS triggered by several fresh fruits, particularly melon, watermelon, banana, cucumber, and apricot.10
Case report
The authors describe the case of a 17-year-old adolescent, followed in Allergy & Clinical Immunology Department since 2010 due to grass pollen-persistent mild allergic rhinitis (according to ARIA Guidelines).11 The patient started oral immunotherapy to grass pollen in November 2011, which was suspended in March 2016, with nasal symptom improvement. In April 2015, she reported mild oral pruritus with onset a few years ago, triggered by apple and pear ingestion. She described progressive symptom worsening with time, with oral allergy symptoms following ingestion of several fresh fruits (banana and kiwi). The girl started to avoid all fresh fruits from April 2015 onwards.
In December 2017, the patient developed oral pruritus during walnut ingestion. Despite having no symptoms, she also started avoiding vegetables in 2015. On regular reassessment in 2017, she had reintroduced vegetables in her diet, with no clinical symptoms. She had no relevant medical or surgical history prior to allergic rhinitis diagnosis. She denied symptoms associated with latex manipulation, had no pets and reported no symptoms when exposed to animal dander. She also denied symptoms associated with other foods and had no family history of allergies.
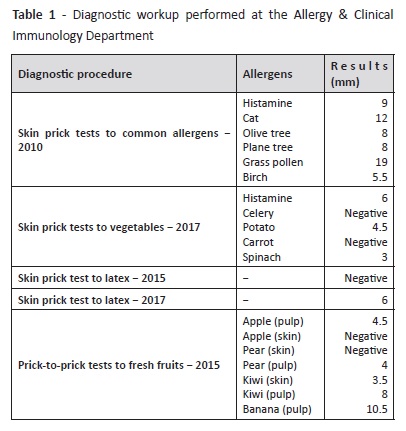
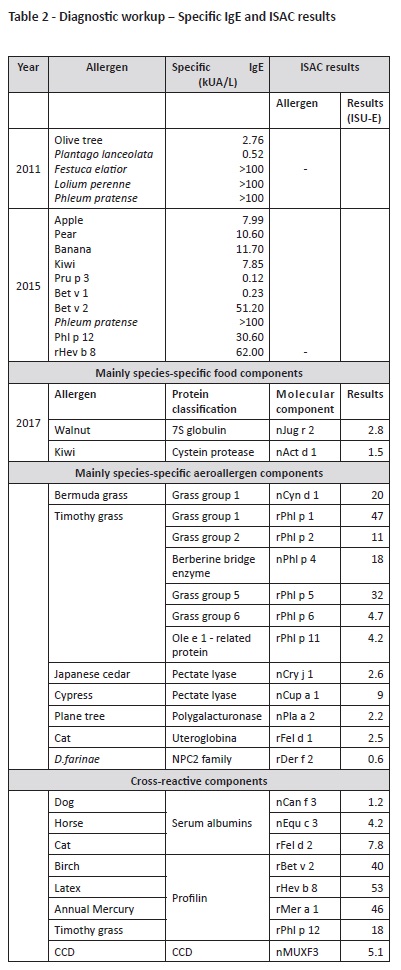
Diagnostic workup performed at our Department is described on Tables 1 and 2. Initial diagnostic procedures included skin prick tests, prick-to-prick tests, and specific IgE. Because the patient was polysensitized and had some dietary restrictions, an Immuno-Solid- Phase Allergen Chip (ISAC) test was performed to clarify the specific molecular allergens involved in the disease and proceed to the safest recommendations on which foods should be avoided. Results are described on Table 2.
According to ISAC test results, a high sensitization to grass pollen due to genuine sensitization allergens (Phl p 1, 2, 5, 6, 11) and cross reactivity (profilins: Phl p 12; CCD-bearing proteins: Cyn d 1, Phl p 4) were observed in this patient. Cupresaceas and plane tree pollen (nCup a 1, nCry j 1 and nPla a 2) also tested positive due to CCD-mediated cross-reactivity. nPhl p 4 and nCyn d 1 are the possible sensitizers of CCD-bearing proteins.
Sensitization to kiwi was also confirmed, due to sensitization to the actinidin Act d 1 and not to profilins. High sensitization to profilins was confirmed, with positive results to all profilins on microarray Bet v 2, Hev b 8, Mer a 1, and Phl p 12. Further positive sensitizations in this patient included CCD and “CCD-bearing proteins” nPhl p 4, nCyn d 1, nCup a 1, nCry j1, nJug r 2, nPla a 2, and MUXF 3. Together with walnut storage protein nJug r 2, these allergens have no clinical relevance when detected; instead, they represent high “in vitro” cross reactivity.12 Sensitization to cat dander was also present (Fel d 1 and Fel d 2), without clinical symptoms.
Discussion
Some aspects are important to reinforce after the present diagnostic workup. Profilin sensitization is associated to OAS with multiple plant-foods and the allergen cross-reacts with multiple pollens. Despite displaying latex sensitization, this patient was only truly sensitized to latex profilin Hev b 8 and not to the major latex allergens, requiring no latex avoidance measures due to absence of clinical significance.13
Another important fact was that kiwi sensitization was not profilin-mediated in this patient. As observed on ISAC test, OAS to kiwi is not due to profilin (Act d 9), but to sensitization to a cysteine protease (Act d 1, actinidin, major allergen of green kiwi pulp), which was not identified in this analysis. Being a major allergen, Act d 1 is associated with kiwi monosensitization and severe systemic reactions. However, it can also be associated with OAS, with cases described in western and central Europe.14
ISAC assessment of other allergens was also important in this clinical case, as it excluded LTPs, PR10, seed storage protein, or thaumatin derived from kiwi fruit.
Despite displaying rFel d 1, rDer f 2, nBos d 6, and nCan f 3 sensitization, the patient had no clinical manifestations of respiratory allergies to house dust mites or animal dander, nor clinical symptoms of food allergy to milk/cow meat.
After a complete diagnostic workup, an oral food challenge with cooked apple was performed (cumulative dose of 140 g), with negative result. Since then, the patient maintains cooked apple ingestion, without symptoms, as well as raw fruit and walnut avoidance. With these adaptations, together with antihistamines and nasal corticosteroid use in the pollen season and emergency medications including antihistamines and systemic corticosteroids, the girl remains asymptomatic.
Conclusions
Similarly to adults, OAS in children and adolescents is commonly triggered by Cucurbitaceae, peach, banana, and kiwi fruit. According to the literature, this endotype is characterized by sensitization to grass, plane tree, and olive tree, as observed in the present clinical case.2 However, even in a “typical” profilin-induced PFS, oral allergy symptoms with fresh fruits are not entirely attributed to profilins. As observed in this specific patient, other allergens are responsible for OAS to kiwi and walnut. Specifically, Act d 1 and CCD seem to be important for OAS clinical symptoms.
As seen in other cases, it is likely that primary sensitization occurred via the respiratory tract, with subsequent OAS. As acknowledged, profilins are labile to pepsin digestion and thermal sensitivity, mainly causing OAS.4 With this in mind, cooked apple was introduced, with good clinical tolerance.
Clinical tolerance to cooked fruits was expected, but the same is not true for kiwi fruit. According to the literature, Act d 1 is a major kiwi fruit allergen, stable to heat and digestion.15 This major allergen can be responsible for oral allergy symptoms, but in some populations it has been associated with severe systemic reactions (particularly in northern Europe).15 This has not been observed in the present patient, who only reported mild oral symptoms. However, the event of a systemic reaction following kiwi ingestion in this patient cannot be ruled out. Despite having only local oral complaints, a systemic reaction is still possible. Therefore, raw and cooked kiwi fruit should be avoided.
The authors further emphasize that different allergens can produce similar symptoms in the same patient, with different consequences. In such cases of multiple sensitization, specific IgE results must be carefully interpreted, as sensitization to profilins, CCD/CCD-bearing proteins, or other allergens may have different clinical implications.
REFERENCES
1. Ludman S, Jafari-Mamaghani M, Ebling R, Fox AT, Lack G, Du Toit G. Pollen food syndrome amongst children with seasonal allergic rhinitis attending allergy clinic. Pediatr Allergy Immunol. 2016; 27:134-40. [ Links ]
2. Mastrorilli C, Tripodi S, Caffarelli C, Perna S, Di Rienzo-Businco A, Sfika I, et al. Endotypes of pollen-food syndrome in children with seasonal allergic rhinoconjunctivitis: a molecular classification. Allergy: European Journal of Allergy and Clinical Immunology. 2016; 71:1181-91. [ Links ]
3. Ta V, Scott D, Chin W, Wineinger N, Kelso J, White A. Differential skin test reactivity to pollens in pollen food allergy syndrome versus allergic rhinitis. Allergy and Asthma Proceedings. 2015; 36: 379-85. [ Links ]
4. Rodríguez del Río P, Díaz-Perales A, Sánchez-García S, Escudero C, Ibáñez MD, Méndez-Brea P, et al. Profilin, a Change in the Paradigm. J Investig Allergol Clin Immunol. 2018; 28:1-12. [ Links ]
5. Hauser M, Egger M, Wallner M, Wopfner N, Schmidt G, Ferreira F. Molecular properties of plant food allergens: a current classification into protein families. The Open Immunology Journal. 2008; 1: 1-12. [ Links ]
6. Nunez R, Carballada F, Lombardero M, Jimeno L, Boquete M. Profilin as a aeroallergen by means of conjunctival allergen challenge with purified date palm profilin. Int Arch Allergy Immunol. 2012; 158:115-9. [ Links ]
7. Ruiz-Garcia M, Garcia del Potro M, Fernandez-Nieto M, Barber D, Jimeno Nogales L, Sastre J. Profilin: a relevant aeroallergen? J Allergy Clin Immunol. 2011; 128:416-8. [ Links ]
8. Santos A, Van Ree R. Profilins: mimickers of allergy or relevant allergens? Int Arch Allergy Immunol. 2011; 155:191-204. [ Links ]
9. Scheurer S, Lauer I, Foetisch K, San Miguel Moncin M, Retzek M, Hartz C, et al. Strong allergenicity of Pru av 3, the lipid transfer protein from cherry, is related to high stability against thermal processing and digestion. J Allergy Clin Immunol. 2004; 114:900-7. [ Links ]
10. Asero R, Tripodi S, Dondi A, Di Rienzo Businco A, Sfika I, Bianchi A, et al. Prevalence and Clinical Relevance of IgE Sensitization to Profilin in Childhood: A Multicenter Study. International Archives of Allergy and Immunology. 2015; 168:25-31. [ Links ]
11. Brozek J, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic- Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. Journal of Allergy and Clinical Immunology. 2017; 140:950-8. [ Links ]
12. Villalta D, Conte M, Asero R, Da Re M, Stella S, Martelli P. Isolated IgE reactivity to native walnut vicilin-like protein (nJug r 2) on ISAC" microarray is due to cross-reactive carbohydrate epitopes. Clin Chem Lab Med. 2013; 51:1991-5. [ Links ]
13. Ebo DG, Hagendorens M, De Knop KJ, Verweij M, Bridts CH, De Clerck LS, et al. Component-resolved diagnosis from latex allergy by microarray. Clin Exp Allergy. 2010; 40: 348-58. [ Links ]
14. Nilsson C, Brostedt P, Hidman J, van Odijk J, Borres P, Sjölander S, et al. Recognition pattern of kiwi seed storage proteins in kiwifruit-allergic children. Pediatric Allergy and Immunology. 2015; 26:817-20. [ Links ]
15. Bublin M, Radauer C, Knulst A, Wagner S, Scheiner O, Mackie AR, et al. Effects of gastrointestinal digestion and heating on the allergenicity of the kiwi allergens Act d 1, actinidin, and Act d 2, a thaumatin-like protein. Mol Nutr Food Res. 2008; 52:1130-9. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Joana Pita
Department of Allergy and Clinical Immunology
Hospital Dona Estefânia
Centro Hospitalar Universitário de Lisboa Central
Rua Jacinta Marto.
1169-045. Lisboa
Email: joana.s.pita@gmail.com
Acknowledgements
To Dr. Leonor Cunha and Professor Moisés Labrador-Horrillo, for their immense collaboration in this work.
Received for publication: 25.11.2018
Accepted in revised form: 07.02.2019