Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Nascer e Crescer
versão impressa ISSN 0872-0754versão On-line ISSN 2183-9417
Nascer e Crescer vol.29 no.3 Porto set. 2020
https://doi.org/10.25753/BirthGrowthMJ.v29.i3.17002
CASE REPORTS | CASOS CLÍNICOS
Chronic spontaneous urticaria in pediatric age
Urticária crónica espontânea em idade pediátrica
Inês Machado CunhaI, Eva GomesI
I Department of Immunoallergology, Centro Hospitalar e Universitário do Porto. 4099-001 Porto, Portugal. inesrjmcunha@gmail.com; evamariasrg@gmx.com
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Introduction: Chronic spontaneous urticaria is characterized by emergence of pruritic maculopapular cutaneous lesions recurring for more than six weeks, without known triggering factor. Association with autoimmunity is sometimes present, with urticaria preceding the onset of autoimmune disease.
Clinical case: A five-year-old female with a personal history of allergic asthma and family history of thyroid disease was referred to the Immunoallergology consultation for cutaneous complaints compatible with urticaria with more than three years of evolution. Inducible urticaria forms were excluded. Analytical study revealed positive antinuclear antibodies with a 1/320 titer and positive basophil activation test after stimulation with autologous serum. Control of cutaneous manifestations was achieved with full dose antihistaminic H1.
Conclusion: Chronic spontaneous urticaria associated with autoimmunity is rare in children. Clinical follow-up should be maintained to evaluate disease control and enable early recognition of other autoimmunity manifestations.
Keywords: autoimmune urticaria; child; chronic spontaneous urticaria; diagnostic investigation
RESUMO
Introdução: A urticária crónica espontânea caracteriza-se pelo aparecimento de lesões cutâneas maculopapulares pruriginosas que recorrem por mais de seis semanas e não têm fator desencadeante. Por vezes encontra-se uma associação a autoimunidade, podendo a urticária preceder o inicio de doença autoimune.
Caso clínico: Uma criança do sexo feminino de cinco anos de idade com antecedentes pessoais de asma alérgica e antecedentes familiares de patologia da tiróide foi referenciada para a consulta de Imunoalergologia devido a queixas cutâneas compatíveis com urticária com três anos de evolução. Foram excluídas formas de urticária indutível. O estudo analítico revelou anticorpos antinucleares positivos com título de 1/320 e teste de ativação de basófilos após estimulação com soro autólogo positivo. Foi alcançado controlo das manifestações cutâneas através de terapêutica com anti-histamínico H1 em dose máxima.
Conclusão: A urticária crónica espontânea autoimune é rara em crianças. O seguimento em consulta permite avaliar o controlo da doença e reconhecer precocemente outras eventuais manifestações de autoimunidade.
Palavras-chave: criança; investigação diagnóstica; urticária autoimune; urticária crónica espontânea
Introduction
Urticaria is characterized by emergence of erythematous maculopapular rash associated with intense pruritus and sometimes burning sensation.1,2 Lesions vanish at digitopression and disappear within twenty-four hours without leaving residual pigmentation.1,2 In 40% of cases, angioedema may appear due to involvement of deeper dermis layers.1,2 Although an underlying allergy is rarely identified, symptoms mainly appear due to specific or nonspecific activation of cutaneous mast cells with subsequent release of histamine and other inflammatory mediators.1,2
Estimates indicate that 20% of the population will suffer at least one urticaria episode.2
Urticaria can be classified according to time course in acute or chronic, depending on symptom persistence beyond six weeks.1,2
In pediatric populations, acute urticaria (AU) is the most frequent form, while chronic urticaria (CU) is rarer, with only 1.8% prevalence.3 In children, the cumulative incidence of all forms of urticaria ranges from 3.8 to 8%.3
Classification suggested by the European Academy of Allergy and Clinical Immunology (EAACI) comprises two subgroups: spontaneous chronic urticaria (SCU), accounting for approximately 75% of cases, and inducible chronic urticaria (ICU), accounting for around 25% of cases.1 The latter can be subdivided into symptomatic dermographism, vibratory angioedema, and cold, heat, sun, aquagenic, cholinergic, and delayed pressure urticaria.1
SCU may be associated with autoimmunity in 40−50% of cases, especially in adult females.1,2
Use of second-generation antihistaminic agents is recommended as initial therapeutic approach due to their favorable safety profile. When using these agents, it is sometimes necessary to use doses four times higher than the usual dose.1,2 In absence of symptomatic control following therapy escalation, immunomodulatory treatment with omalizumab (approved in patients with ≥12 years) or cyclosporine A may be considered.1,2 Although there is no accurate data regarding CU resolution in pediatric age, studies in the adult population indicate spontaneous resolution in five years in 30−55% of cases.4-6 Factors that have been reported as potentially influencing prognosis include Urticaria Activity Score 7 (UAS 7) questionnaire gender, age of symptom onset, number of peripheral basophils in blood, degree of basophil activation in autologous serum activation test, and antihistamine dose required for disease control.7-10 Evaluation of these parameters may help predict the natural course of disease.
Herein is reported a case of SCU associated with autoimmunity in pediatric age, a rare condition in childhood requiring special attention.
Case report
A five-year-old girl was sent to the Immunoallergology consultation due to recurrent pruritic maculopapular skin eruption with one year of evolution. No intercurrences in prenatal or neonatal period were reported. She had a personal history of recurrent wheezing treated with Montelukast 5 mg per day and maternal family history of thyroid pathology and allergic rhinitis. The girl was in the 25−75% weight percentile and in the 25−50% height percentile, with age-adapted psychomotor development. She reported no known drug, food, or aeroallergen allergies.
On initial evaluation, the patient reported maculopapular, erythematous, and pruritic skin lesions at least twice a week, with each lesion lasting less than twenty-four hours. No episodes of angioedema were described and the patient denied fever, weight loss, asthenia, photophobia or ocular pain, osteoarticular or muscular complaints, mucosal ulcerations, xerostomia or intestinal transit changes, or other associated symptoms. No relation with physical factors as cold, hot, water, vibration, or sun exposure was identified, and no specific or non-specific aggravating factors − like drugs, food, or exercise − were suspected. The girl was already receiving levocetirizine 1.25 mg per day, prescribed by the attending physician. Disease activity was assessed by UAS 7 filling, with a total score of nine, suggestive of uncontrolled mild urticaria. She also reported nasal obstruction, mucosal rhinorrhea, and dry cough during the night, exacerbated by physical exercise, and denied dyspnea, wheezing, or chest pain.
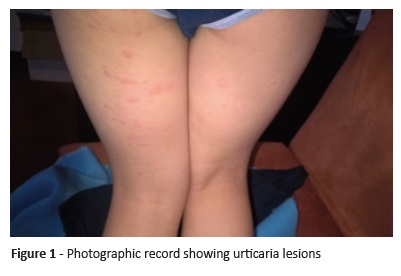
On physical examination, the patient revealed constitutional facial erythema. Oropharynx, nasal cavity, and pulmonary exams were normal. There were no visible urticarial lesions, pruritus lesions, or dermographism. The mother presented photographic documentation of skin lesions compatible with urticarial wheals (Figure 1).
Based on clinical data, uncontrolled chronic urticaria and uncontrolled bronchial asthma were proposed as probable diagnoses. Complementary analytical study and pulmonary function test were requested. Levocetirizine dose was adjusted to 1.25 mg twice daily and the patient started fluticasone 125 ug once daily on a spacer, salbutamol 100 ug as SOS on a spacer, and nasal topical corticosteroid therapy. Montelukast was suspended.
On second follow-up visit, six weeks later, the patient reported only one urticaria episode. She reported no respiratory pathology exacerbations and did not resort to rescue medication (salbutamol) in any occasion, although maintaining dry cough with exercise. She denied new symptoms. As UAS7 was zero, the disease was considered under control.
Analytical study showed absence of basophils in the peripheral blood, positive anti-nuclear antibodies (ANA) with titer of 1/320 (normal reference value <1/80), and basophil degranulation test with autologous serum with 6% of basophil activation, findings compatible with autoimmune urticaria diagnosis. The patient did not cooperate on pulmonary function evaluation.
Other studies performed − hemogram with platelets, sedimentation rate, C-reactive protein, biochemistry with glucose, renal function, hepatic function, thyroid function, sediment and urinalysis, complement study, and Phadiatop − showed no changes. Autoimmunity study, including anti-thyroglobulin, anti-peroxidase, anti-DsDNA, anti-Sm, anti-U1 RNP, anti-SSA, anti-SSB, and rheumatoid factor, was also negative.
In the next appointment, the girl reported monthly urticaria episodes lasting between three to seven days and levocetirizine dose was increased to 2.5 mg twice daily.
With a two-year follow-up, she maintains levoceterizine 2.5 mg twice daily, fluticasone 125 ug twice daily, and salbutamol 100 ug as SOS. Analytical study was repeated, with results similar to the former. The girl currently maintains regular follow-up in the Immunoallergology department.
Discussion
This study describes the case of a female child with CU. In the investigation, it is important to exclude differential diagnoses, such as autoinflammatory syndromes (Schnitzler syndrome and periodic syndrome associated with cryopyrin), and identify possible precipitating factors in order to distinguish between SCU and ICU.1,2
Patient’s clinical history did not allow to identify a physical triggering factor, excluding vibratory angioedema symptomatic dermographism, cold, heat, pressure, solar, aquagenic, cholinergic, and contact urticaria.
ANAs are reported in 0−29% of CU patients.2 Although their identification alone may not have pathological significance, when associated with urticaria, patients should be periodically evaluated, as SCU may be the initial manifestation of systemic or organ-specific autoimmune diseases and precede development of an autoimmune disease for several years.11-14
Second-generation antihistamines are recommended as first-line pharmacological treatment due to their favorable safety profile.1,2,19,20 Symptomatic control should be evaluated four weeks after the initial proposed treatment and dose increased to up four times the standard dose if needed.1,2
In this patient, basophil degranulation test with autologous serum was positive, with CD63 expression increase in 6% of cells. As this test is done with autologous serum, anti-FcεRIα and/or anti-IgE antibodies are considered capable of inducing basophil activation and degranulation by autoimmunity phenomena.1518
The natural course of disease in pediatric age is unknown, with some studies reporting a urticaria remission rate approaching 32% in one year and reaching 50% in five years.10 Probability of remission appears to be lower when circulating basophils are low, UAS7 is greater than 28, and there is a need for therapy with high antihistamine doses, as well as in female children over the age of ten years.7,8,10 Some data suggests that basophil activation test with values above 1.8% is indicative of a better prognosis, increasing to double the probability of symptom resolution after one year of disease.9
In the present case, the child presented basophil values within the expected, low UAS7 score, and a degree of basophil activation higher than 1.8%, but high doses of antihistaminic were still necessary to control the disease.
Conclusions
SCU may have an autoimmune cause, be associated with autoimmune disease, or precede subsequent autoimmune disease. Disease evolution is not predictable, although series in the literature report resolution in approximately 50% of cases. Follow-up should be maintained until resolution. In the case of analytical results suggestive of autoimmune disease, follow-up in consultation allow early identification of possible onset of other pathologies, in addition to disease control monitoring and eventual therapeutic adjustments.
REFERENCES
1. Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. Allergy. 2018; 73:1393-414. [ Links ]
2. Costa C, Gonçalo M. Abordagem diagnóstica e terapêutica da urticária crónica espontânea: recomendações em Portugal. Acta Med Port. 2016; 29:763-81. [ Links ]
3. Brüske I, Standl M, Weidinger S, Klümper C, Hoffmann B, Schaaf B, et al. Epidemiology of urticaria in infants and young children in Germany-results from German LISAplus and GINIplus Birth Cohort Studies. Pediatr Allergy Immunol. 2014; 25:36-42. [ Links ]
4. Sahiner UM, Civelek E, Tuncer A, Tolga Yavuz S, Karabulut E, Sackesen C, et al. Chronic urticaria: etiology and natural course in children. Int Arch Allergy Immunol. 2011; 156:224-30. [ Links ]
5. Yilmaz E, Karaatmaca B, Cetinkaya P, Soyer O, Bulent E, Sahiner U. The persistence of chronic spontaneous urticaria in childhood is associated with the urticaria activity score. Allergy Asthma. 2017; 38:136-48. [ Links ]
6. Netchiporouk E, Sasseville D, Moreau L, Habel Y, Rahme E, Ben-Shoshan M. Evaluating Comorbidities, Natural History, and Predictorsof Early Resolution in a Cohort of Children With Chronic Urticaria. Jama Dermatol. 2017; 153:1236-42. [ Links ]
7. Eser I , Yologlu N, Baydemir C, Aydogan M. The predictive factors for remission of chronic spontaneous urticaria in childhood: Outcome from a prospective study. Allergol Immunopathol. 2016; 44:537-41. [ Links ]
8. Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013; 68:27-36. [ Links ]
9. Dalal I, Levine A, Somekh E, Mizrahi A, Hanukoglu A. Chronic urticaria in children: Expanding the “autoimmune kaleidoscope.” Pediatrics. 2000; 106:1139-41. [ Links ]
10. Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012; 129:1307-13. [ Links ]
11. Szegedi A, Irinyi B, Gal M, Knol EF, Maurer M, Raap U, et al. Significant correlation between the CD63 assay and the histamine release assay in chronic urticaria. Br J Dermatol. 2006; 155:67-75. [ Links ]
12. Gyimesi E, Sipko S, Danko K, Kiss E, Hídvégi B, Gál M, et al. Basophil CD63 expression assay on highly sensitized atopic donor leucocytes-a useful method in chronic autoimmune urticaria. Br J Dermatol. 2004; 151: 388 -96. [ Links ]
13. Fortina AB, Fontana E. Update on antihistamine treatment for chronic urticaria in children. Curr Treat Opt Allergy. 2014; 1:287-98. [ Links ]
14. Boguniewicz M. The autoimmune nature of chronic urticarial. Allergy Asthma Proc. 2008; 29:433-8. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Inês Machado Cunha
Department of Immunoallergology
Centro Hospitalar e Universitário do Porto
Largo Professor Abel Salazar
4099-001 Porto
Email: inesrjmcunha@gmail.com
Received for publication: 01.02.2019. Accepted in revised form: 13.11.2019