Introduction
Congenital pulmonary airway malformation (CPAM) and bronchopulmonary sequestration (BPS) are rare congenital anomalies of unclarified etiology. BPS corresponds to 0.15−6.45% of all pulmonary malformations.1,2 Identified in 1946 by Pryce, the condition is characterized by presence of non-functioning pulmonary parenchyma, without communication with the bronchial tree, with arterial supply originating from systemic circulation, usually from the aorta.3,4BPS can be further classified into “intralobar sequestration” (ILS) and “extralobar sequestration (ELS), depending on whether the sequestered tissue is contained within a normal pulmonary lobe or separated from the pulmonary lobe with its own pleural lining, respectively. During prenatal period, it can be diagnosed from the 16th week of gestation by obstetric ultrasonography.5
CPAM, previously named congenital cystic adenomatoid malformation by Ch’in and Tang in 1949, is the most common pulmonary malformation (30−40% of patients), with a prevalence of 1 in 10,000−35,000 births.2,6,7 It consists of a pulmonary maturation anomaly, resulting in formation of multiple cysts of gaseous or liquid content with varying size and distribution. CPAM was classified in 1977 by Stocker in three categories based on embryonic origin and histological characteristics, with two categories subsequently added. CPAM type 0 is the rarest form (1−3%), characterized by small cysts (0.5 cm) affecting the trachea or bronchi and presenting in a severe and fatal way at birth. CPAM type 1 is the most common form (60−65%), with multiple cysts of 10 cm in diameter reaching the distal bronchi or the proximal bronchioles. CPAM type 2 corresponds to 15−20% of cases, originates in the terminal bronchioles, and is characterized by multiple cysts of 0.5−2 cm in diameter. CPAM type 3 is less frequent (5−10%) and consists of solid-aspect lesions involving one or more pulmonary lobes, with multiple cysts of less than 0.5 cm in diameter which, by mass effect, may result in dyspnea and subsequent neonatal death. CPAM type 4 accounts for 5−10% of cases, originates in the alveoli, and consists of lesions with a maximum diameter of 7 cm manifesting as pneumothorax or pneumonia and having significant carcinogenic potential.1,2,8,9 In 15−20% of cases, CPAM is associated with other congenital malformations, mainly cardiac and renal.9 Diagnosis can be performed between the 21st and 24th weeks of gestation and the condition may stabilize by the 28th week of gestation or spontaneously regress.7,10 CPAM and BPS are usually asymptomatic during prenatal and neonatal periods, but fetal hydropsia or respiratory distress syndrome may occur after birth.5,10
Case report
A two-month-and-three-week-old full-term female infant with an uneventful pregnancy, with prenatal ultrasounds described as normal, was suspected of cystic fibrosis in the Guthrie test, not confirmed in the sweat test. The girl presented recurrent respiratory infections since the third week of life. Following radiologically confirmed pneumonia diagnosis, she was medicated with oral amoxicillin and clarithromycin for ten days. Four days after treatment discontinuation, patient’s clinical condition worsened, with fever, cough, and dyspnea, and she was admitted to the Pediatric Urgency Department. Clinical examination revealed tachycardia (177 beats per minute), polypnea (64 breaths per minute), subcostal retractions, intermittent grunting, peripheral oxygen saturation of 94%, scattered late inspiratory crackles, and decreased vesicular murmur in the left pulmonary base.
Routine investigation revealed C-reactive protein of 147.6 mg/L, leukocytes of 5.2 x 109 cells/L (61.8% neutrophils), and negative blood culture. Chest radiography showed a heterogenous hypotransparent image in the lower two thirds of the left pulmonary field and mediastinal shift to the right hemithorax (Figure I). Pneumonia of bacterial etiology was assumed and the patient was treated with cefotaxime.
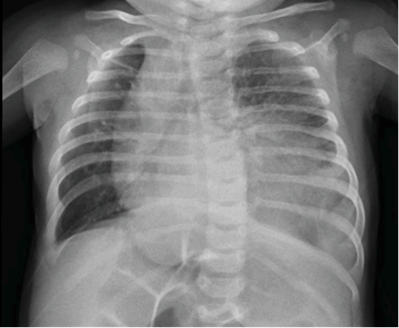
Figure I Chest radiography showing heterogenous hypotransparent image in the lower two thirds of the left pulmonary field and mediastinal shift to the right hemythorax
As radiological alterations persisted between chest radiographs, thoracic computed tomography (CT) was performed, disclosing a heterogenous mass in the left lower lobe with hypodense cystic areas (dimensions ≤10 mm) and mediastinal shift to the right hemithorax, suggestive of CPAM. A similar smaller lesion was also identified in the paramediastinic right lower lobe. Angiotomography was performed, revealing bilateral BPS with left lower lobe destruction (Figure II), a systemic vessel originating in the abdominal aorta and irrigating the area, and a cystic lesion in the right lower lobe (Figures IIIandIV).
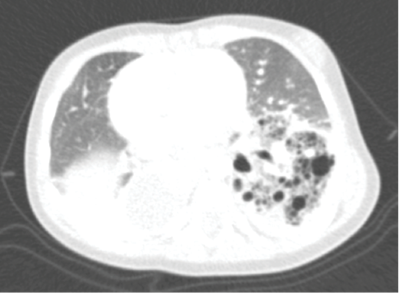
Figure II Thoracic angiotomography showing bilateral bronchopulmonary sequestration with lower left lobe destruction
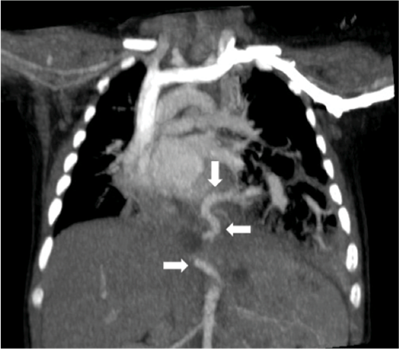
Figure III Thoracic angiotomography showing anomalous vessel originating in the abdominal aorta (arrows)
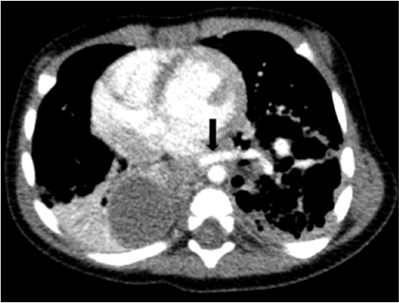
Figure IV Thoracic angiotomography showing anomalous vessel originating in the abdominal aorta (arrow)
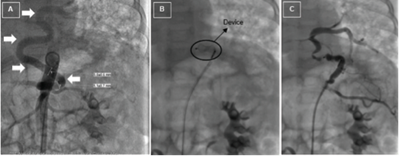
The patient was submitted to catheterization with occlusion of the anomalous vessel with an 8-mm Amplatzer Vascular Plug device (Abbott Vascular, Santa Clara, California) at the age of seven months (Figure V). Two months later, the vessel was lacquered and left lower lobectomy was performed. Histological examination revealed ILS and associated CPAM type 2, and several bronchiectases filled with thick yellow material were identified. At 14 months, the patient was submitted to resection of the right lower lobe segment and histological study revealed ELS associated with hamartomatous lung malformation. Clinical outcome was favorable and the patient remained asymptomatic.
Discussion
Similarly to the present case, hybrid lesions sharing BPS and CPAM characteristics have been described in the literature.11 No chromosomal abnormalities are known and the pathophysiology is not completely clarified, but it is hypothesized that BPS and CPAM share a common embryological origin, particularly CPAM type 2 and ELS.1,2,9-11
ELS is less frequent than ILS (75% of cases) and usually located in the left lower lung lobes (80%).1 In the present case, ELS was reported on the right lung. ILS is mainly located in the left lower lobe, as in the present case, and less frequently associated with other congenital malformations (15%) than ELS, which has been associated with cardiac and chest wall, diaphragmatic, or other pulmonary anomalies, such as CPAM type 2 (up to 50% of cases).1,4,9,12 However, association of ILS with CPAM type 2 is rare, as well as occurrence of both BPS forms simultaneously, as in this case.1,13 Postnatal BPS diagnosis is established by angiotomography, and CPAM as well as bronchogenic or enteric duplication cysts, among other lesions, should be considered in the differential diagnosis.1,5 ILS mainly presents through recurrent respiratory infections, while ELS is often asymptomatic.1,5,13
CPAM type 2 can manifest from the first month of life, mainly due to recurrent respiratory infections, and most often affects the lower lobes, as observed in the present case.1,14 This is the CPAM subtype most often associated with other congenital malformations, such as cardiac, renal, esophageal or intestinal atresia, and skeletal alterations, among others, and prognosis depends on these findings, which were not found in the present patient.2,9 A total of 30% of cases are only diagnosed during the postnatal period through contrast-CT; this may occur if cystic lesions are collapsed during the prenatal period, allowing normal development of the remaining pulmonary lobes.1,7,15 BPS, bronchogenic cysts, congenital lobar emphysema, cystic fibrosis, and congenital diaphragmatic hernia, among others, should be considered in the differential diagnosis.2,7,9,15 Exclusion of cystic fibrosis was crucial in the present case, as it can present with recurrent respiratory infections and bronchiectasis.2 When symptomatic during the postnatal period, CPAM mainly presents with recurrent respiratory infections, as in the present case, but may also present with pulmonary hemorrhage or pneumothorax, and malignant transformation can also occur.1,14
In symptomatic cases of BPS and CPAM, as the one reported, surgical resection should be performed through sequestrectomy or pulmonary lobectomy.1,5 Identification, occlusion, and lacquering of the anomalous vessel irrigating BPS is crucial to decrease the hemorrhagic risk.5,9 Risk of malignant transformation and recurrent respiratory infections are factors favoring surgical treatment in asymptomatic CPAM or BPS cases.1,5,7,9,10
This case illustrates the importance of maintaining a high index of suspicion of congenital anomalies in infants with recurrent respiratory infections and persistent radiological alterations. Surgical resection allowed definitive treatment and confirmed diagnosis.