Introduction
Respiratory distress syndrome (RDS) is a common morbidity and mortality cause in preterm infants.1,2 Surfactant therapy is a hallmark in RDS treatment, being associated with an important decrease in preterm mortality.
For many years, surfactant therapy was administered to sedated and intubated patients, with the disadvantages of mechanical ventilation: volume and barotrauma, atelectrauma, and biotrauma. To reduce these complications, Victorin et al developed the INSURE technique in the 1990s.3 The name INSURE is an abbreviation for INtubation-SURfactant-Extubation and refers to the procedure by which a patient on nasal continuous positive airway pressure (nCPAP) ventilation is intubated, receives surfactant through an endotracheal tube, and is extubated back to nCPAP. This procedure has advantages over the traditional surfactant administration, including mortality decrease, mainly due to a reduction in the use of invasive ventilation. Despite these advantages, INSURE also has disadvantages, such as frequently requiring sedation, with potential secondary effects, as bradycardia or hypotension, and extubation difficulty in a large number of patients.4
Several non-invasive or less invasive techniques for surfactant administration in non-intubated patients have been developed over time to avoid intubation-associated risks, namely administration via thin catheter (the most studied and employed method), aerosolized administration, laryngeal mask-guided administration, and pharyngeal administration.1 This group of techniques has been inconsistently designated minimally invasive surfactant therapy (MIST) or less invasive surfactant administration (LISA). In this study, the first designation was adopted.
The most frequently described method for surfactant administration via thin catheter is through a feeding tube, known as the Cologne method. It was first described by Verder et al5 and uses a 4- to 5-FG feeding tube and Magill forceps to introduce a thin catheter past the vocal cords. This technique allows surfactant administration while the patient is on non-invasive positive pressure ventilation.4
In recent years, several small trials have encouraged the use of MIST over the conventional INSURE technique6-11 and larger studies (NINSAP and OPTIMIST-A) have also investigated the benefits of this procedure. In NINSAP trial, no difference was found between both methods regarding the primary outcome of death or progression to bronchopulmonary dysplasia (BPD) and some secondary outcomes, as intraventricular hemorrhage and pneumothorax, were significantly reduced with MIST. OPTIMIST-A is a large ongoing multicentre randomized controlled trial (RCT) that aims to investigate progression to BPD or death in a patient population with surfactant administration by MIST (Hobart method).4
A recent meta-analysis of three RCTs comparing MIST with INSURE showed that the minimally invasive technique reduced the need for mechanical ventilation, nCPAP duration, oxygen supplementation, and progression to BPD compared with INSURE.12
The aim of this study was to investigate the efficacy and feasibility of MIST compared with INSURE and assess short- and long-term effects, including intubation, oxygen supplementation, inward duration, and procedure complications.
Material and methods
Patients
This was a retrospective observational study conducted in the Neonatal Intensive Care Unit (NICU) of Centro Materno-Infantil do Norte, Centro Hospitalar Universitário do Porto, between January 1st 2015 and June 30th 2019.
Inclusion criteria comprised preterm infants admitted to NICU who received nCPAP support and surfactant (Curosurf®, Chiesi Farmaceutici, Parma, Italy) either by INSURE or MIST technique.
MIST procedure
MIST was performed in patients on nCPAP with a fraction of inspired oxygen (FiO2) equal or above 0.3. Direct laryngoscopy was performed and a 5-FG feeding tube was inserted into the trachea with Magyll forceps. Surfactant was instilled through the feeding tube at standard dose (200 mg/Kg) as a slow bolus and the catheter immediately removed. Analgesia with sucrose and/or morphine was frequently administered before the procedure. Positive pressure inflation was provided in cases of apnea and/or bradycardia.
INSURE procedure
INSURE was performed via elective intubation and surfactant was given via endotracheal tube (ET) while on positive pressure ventilation without target volume. After administration, ET was promptly removed and the patient returned to nCPAP. Patients who did not respond started invasive ventilation.
Statistical analysis
Patient data analysed included gender, gestational age, birth weight, prenatal corticosteroid administration, and pre- and post-surfactant FiO2. Complications, oxygen therapy duration, respiratory support (nCPAP and/or invasive ventilation), and NICU length of stay were also recorded.
Sociodemographic data were expressed as medians. Discrete variables were analyzed with Chi-square test. Depending on data distribution, Student’s t or Mann-Whitney U test were performed to compare continuous variables. Statistical analysis was performed using IBM® SPSS® Statistics, version 25.0 (SPSS, Chicago, IL, USA).
Results
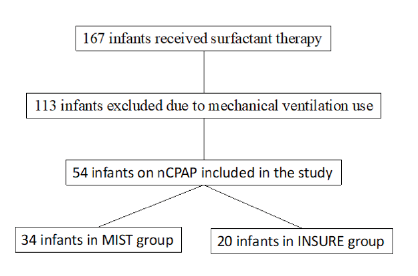
During the study period, 167 neonatal preterm infants required surfactant therapy in NICU. A total of 113 patients were excluded from the study for having received surfactant under invasive ventilation. Overall, 54 patients were treated with nCPAP, 34 of which by MIST (Group 1) and 20 by INSURE (Group 2; Figure 1). Both techniques were performed by NICU neonatologists.
Group 1 included 15 female and 19 male patients versus 6 and 14 in Group 2, respectively. Demographics and general characteristics, as gestational age (p=0.480), birth weight (p=0.299), and median maternal age (p=0.129), were balanced between groups, as were prenatal corticosteroid administration (p=0.395), intrauterine growth restriction incidence (p=0.147), delivery mode (p=1.000), and median Apgar Score in first and fifth minutes (p=0.705 and p=0.902, respectively). Birthweight class analysis also showed no relevant between-group differences (Table 1).
Table 1 General characteristics of the study population

IQR, interquartile range; IUGR, intrauterine growth restriction; ST, surfactant therapy
* Fisher’s Exact test
** Mann-Whitney U test
According to medical records, 14 patients received premedication, all from MIST group. Five patients received morphine (0.05 to 0.1mg/Kg) and atropine (0.01 to 0.02mg/Kg), seven only morphine, one morphine and midazolam, and one only atropine.
Table 2 depicts FiO2 values before surfactant administration and respective variation before and after the procedure. No significant differences were found between Group 1 and 2 regarding FiO2 before surfactant (p=0.220) and FiO2 variation before and after the procedure (p=0.078).
Pneumothorax was reported in two MIST patients (6%), but deemed to be unrelated to the procedure. Regarding complications directly related to the procedure, gastric surfactant deposition was reported in three MIST patients (9%). MIST was repeated in four patients (12%) and INSURE in three (15%). Five MIST patients (15%) compared to four INSURE patients (20%) required mechanical ventilation within the first 72 hours after surfactant administration. General characteristics were balanced and not significantly different between both cohorts (Table 2). Among MIST patients, one was intubated three hours after surfactant administration due to apnea with bradycardia episodes. Information about reasons for intubation was lacking in the medical records of the remaining MIST patients. In INSURE group, one patient was intubated due to pneumothorax and another due to apnea with bradycardia. In the latter, surfactant was observed in gastric aspirate. Data was lacking for the remaining two INSURE patients.
Median oxygen therapy duration was 7.5 days (interquartile range [IQR] 33) in Group 1 and 25 days (IQR 34) in Group 2 and median NICU length of stay was 15 days (IQR 23) and 13 days (IQR 23), respectively. No statistically significant differences were found between groups regarding oxygen therapy duration (p=0.778) and NICU length of stay (p=0.942).
Prevalence of bronchopulmonary dysplasia was similar with both techniques, with 12 cases reported in MIST and seven reported in INSURE group (both 35%). Other clinical complications are described in Table 3. Prevalence of hemodynamically significant patent ductus arteriosus (HS-PDA) (five in MIST vs four in INSURE) and severe intraventricular hemorrhage and cystic periventricular leukomalacia (three in MIST vs two in INSURE) was similar between groups. Retinopathy of prematurity (ROP) and necrotizing enterocolitis (NEC) were only found in Group-1 patients (three and one cases, respectively).
One MIST patient died of reasons unrelated to the procedure.
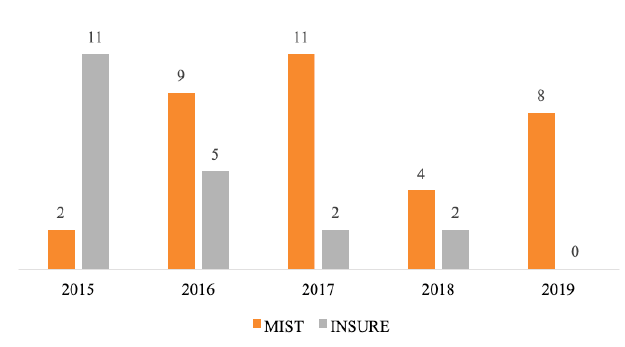
An increased interest in surfactant administration via catheter has been observed in NICU since the beginning of MIST use, with a total of 15% surfactant administrations in 2015 and 100% in the first half of 2019 (Figure 2).
Table 2 Clinical characteristics of the study population
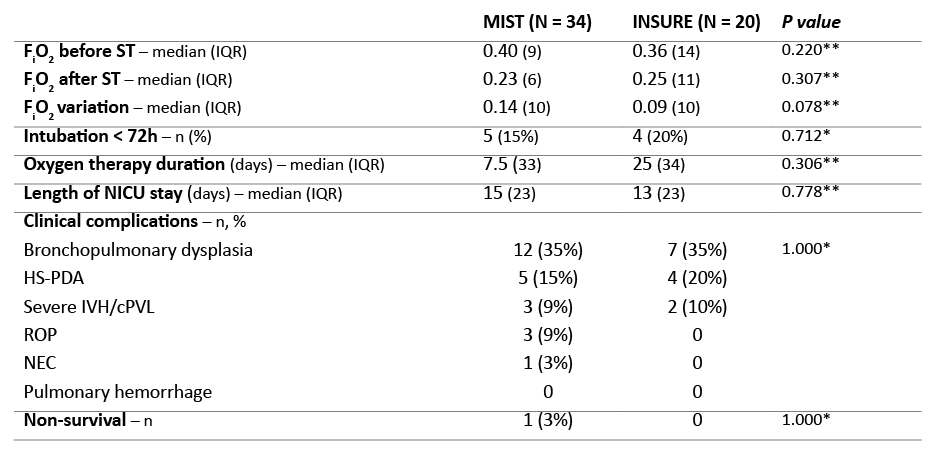
cPVL, cystic periventricular leukomalacia; FiO2, fraction of inspired oxygen; HS-PDA, hemodynamically significant patent ductus arteriosus; IQR, interquartile range; IVG, intraventricular hemorrhage; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; ST, surfactant therapy
* Fisher’s Exact test
** Mann-Whitney U test
Table 3 General characteristics of patients intubated within the first 72 hours
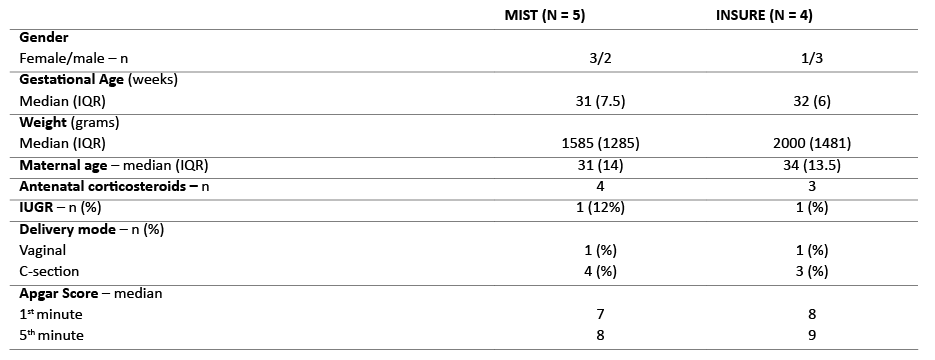
IQR, interquartile range; IUGR, intrauterine growth restriction; ST, surfactant therapy
Discussion
Avoiding mechanical ventilation has been the clinical focus in RDS preterm neonates in recent years. Several large clinical trials (COIN, SUPPORT, and VON-DRM) reported no benefit with INSURE over only nCPAP support.13-15 This may be explained by adverse effects associated with intubation and invasive ventilation, which may increase acute lung injury in preterm patients. Other disadvantages are related to the need for sedative medication (with associated secondary effects, as bradycardia and hypotension) and extubation difficulty.4 Patients submitted to INSURE often fail to be extubated after surfactant administration, resulting in longer mechanical ventilation support. In a 2014 cohort study, 60% of patients treated with this technique failed to be extubated in the first two hours after surfactant administration.16
Additionally, not all preterm infants are effectively managed with nCPAP only. For some neonates with moderate-to-severe RDS, nCPAP support seems insufficient, and more aggressive respiratory management is required.4 In this setting, minimally invasive surfactant therapy techniques emerged as a strategy for avoiding invasive ventilation in these patients.
MIST enables surfactant administration while the patient is on noninvasive CPAP. Four different MIST methods have been described: intrapharyngeal surfactant instillation (first used by Enhoerning and Robertson in 1972 in a rabbit model); surfactant nebulization (a promising procedure also known as noninvasive surfactant therapy, but with several physical limitations related to aerosol size and nebulizer type); surfactant instillation via laryngeal mask; and surfactant administration via thin catheter.16-18
Surfactant administration via thin catheter can be performed by four different methods, with the Cologne method being the most widely used. It was first described by Verder et al and published by Kribs et al in 2007.19 Hobart method is an alternative procedure described in 2011 which obviates the need for Magill forceps and is currently being investigated in the large OPTIMIST-A trial.20 This multicentre RCT is enrolling preterm infants with 25−28 weeks of gestation with six hours of life treated with nCPAP and with FiO2 ≥0.30 and aims to compare surfactant administration via Hobart method (intervention group) versus via nCPAP (control group).
No study has been conducted to date comparing MIST methods, which results in great clinical practice heterogeneity within NICUs worldwide.
INSURE remains the most commonly used surfactant administration method in several Portuguese NICUs. In our NICU, INSURE is the chosen method when there is the possibility of patients requiring invasive ventilation.
In the present study, both INSURE and MIST patient populations displayed similar general demographic characteristics and median FiO2 before surfactant administration. Progression to mechanical ventilation, oxygen therapy duration, and length of NICU stay were also similar between groups. Although data was lacking for some patients, reasons for intubation in the first 72 hours were similar between groups, with one apnea and bradycardia episode reported in one patient in each group. Although not statistically significant, the most relevant between-group difference was the greater decrease in FiO2 requirements after surfactant administration with MIST compared with INSURE (0.14 vs. 0.09, p=0.078).
MIST also has associated issues. Preterm infants with mild RDS probably improve with nCPAP and do not benefit from MIST. MIST comprises several methods and techniques and successful outcomes are directly related to staff experience and training. In our NICU, method success has progressively increased over the last four years due to staff-acquired skills.
Premedication is another issue to take into consideration. Premedications may include oral sucrose, atropine, ketamine, caffeine, morphine, lidocaine, among others. A 2017 European survey reported that 52% of neonatologists used no premedication in MIST. Use of narcotic agents is common in INSURE, but their absence does not seem to be associated with short-term deleterious effects. Since spontaneous breathing plays a major role in surfactant pulmonary distribution in MIST, breathing effort reduction with narcotics may be disadvantageous in preterm infants.21 In our NICU, sucrose, low morphine doses, and in some cases atropine are usually used in MIST. However, information regarding premedication is lacking in our database, what constitutes a study limitation; this information was only available for 14 of the total enrolled patients.
As surfactant reflux is a common complication, the effective dose administered is difficult to determine.22 In this study, this complication was reported in three cases, but its importance is difficult to discuss due to limitations associated with the study’s retrospective design. Other complications associated with the technique, like unilateral surfactant deposition, mucosal bleeding, or airway obstruction, were not observed.
Although no side effects were observed with MIST in this study, the procedure may pose technical challenges, like using a laryngoscope to visualize vocal cords, what can be difficult and sometimes traumatic.23
Due to the retrospective nature of this study, information gaps in clinical records preclude conclusions regarding aspects as the effective surfactant dose, premedication, and minor technique complications.
Previous Australian and European studies have shown a growing interest of neonatologists in MIST methods.24 In the 2019 European Consensus Guidelines on RDS management, less invasive surfactant administration is recommended as the preferred surfactant administration method for spontaneously breathing infants on nCPAP, provided that clinicians are experienced with the technique (B2).25 This study supports these results, with MIST being the preferred technique by most neonatologists in our NICU.
Conclusions
In the literature, MIST is associated with a significant reduction in mechanical ventilation requirement and duration, supplemental oxygen, and nCPAP. This means that surfactant administration via thin catheter may have a role in the future care of preterm infants.12
This study confirms some of the potential advantages of minimally invasive surfactant administration reported in literature.
MIST via catheter is a gentle, feasible, and effective technique in preterm RDS infants. This technique is preferred over surfactant administration via INSURE due to inherent intubation disadvantages in preterm neonates with moderate-to-severe RDS.
Some MIST-associated problems should be emphasized, as method variability (with potential result discrepancy), uncertainty about the effective surfactant dose, premedication use, and patient selection. Overall, MIST seems to be as effective as INSURE, but the best minimally invasive method remains to be determined. Further studies are required to standardise indications and procedures, namely comparing the four different MIST techniques.