Introduction
Egg allergy represents the second most common cause of food allergy in childhood. Its prevalence in children is estimated to range between 0.5─3.5%,1-5 with most children acquiring tolerance between the ages of two and five years.4,6
Measles, mumps, and rubella (MMR) vaccination is pivotal for the primary prevention of those infectious diseases, diminishing the probability of infection and mortality. MMR vaccine contains in its formulation live attenuated viruses grown in cultured chick embryo fibroblasts, raising concerns about the presence of ovalbumin, the most abundant (>50%) allergenic protein in the egg white. However, because it is only partially stable to cooking and unstable to digestive enzymes, ovalbumin is little allergenic. The amount of ovalbumin present in the vaccine is considered insufficient to cause an allergic reaction, and other vaccine constituents are believed to be responsible for the adverse reactions seen after administration.2,4,6-13
Although the incidence of anaphylactic reactions to MMR vaccine is very low and studies show its safety in children allergic to egg, physicians still have concerns regarding its inoculation, which often results in delays in vaccine administration, with associated risks.2,4
The aim of this study was to characterize the pediatric population referred to the Allergy and Clinical Immunology Department for administration of the MMR vaccine and its safety in children with egg allergy or egg sensitization.
Material and methods
This was a retrospective study of all patients with a history of egg allergy or egg sensitization requiring MMR vaccination attended at the Allergy and Clinical Immunology Department between January 1, 2013 and December 31, 2019.
The following data were retrieved from patients’ clinical records: demographics; age of MMR immunization; egg allergy symptoms; history of atopy; results of skin prick tests (SPT) with egg extract; total immunoglobulin E (IgE) titers; titer of serum-specific IgE against egg white, egg yolk, ovalbumin, and ovomucoid; result of oral food challenge (OFC) with egg; adverse reactions after MMR vaccine administration; setting where immunization took place (hospital or primary health care center).
The study sample was divided into two groups: Group A, including all patients who received the first dose of MMR vaccine, and Group B, including all patients who received the second dose of MMR vaccine.
Data was analyzed through descriptive statistics, using mean and standard deviation for quantitative data and frequency expressed as percentage for qualitative data.
Results
A total of 60 children were included during the considered time period, 46 (76.7%) male. Vaccination occurred in hospital setting in all cases. Forty-eight children had received the first administration of the MMR vaccine.
Most children (90%) presented clinical history suggestive of egg allergy, with 40 children presenting with cutaneous symptoms, seven with gastrointestinal symptoms, three with respiratory symptoms, and four with egg anaphylaxis history. Among the remaining six children (10%), five had never ingested egg at the time of referral, and one had an unknown time of egg introduction in the diet.
In the five children who had never ingested egg, the motive for referral was cow’s milk protein allergy and parental refusal of immunization in the primary care setting. However, these children had egg introduced in their diet during follow-up, developing cutaneous symptoms upon egg ingestion. One of them had total serum IgE and specific IgE within the normal range, negative SPT, and negative OFC with egg, excluding the diagnosis of egg allergy. The other four patients showed high total serum IgE and positive IgEs specific for egg white, egg yolk, ovalbumin, or ovomucoid, as well as positive OFC with egg. The child with unknown timing of egg introduction in the diet had been referred due to a reaction following MMR immunization at the age of 15 months. After medical history assessment (local cutaneous reaction), an allergic reaction to MMR vaccine was excluded. The child showed total serum IgE within the normal range, specific IgEs for egg yolk, egg white, ovomucoid, and ovalbumin, and negative OFC, excluding egg allergy.
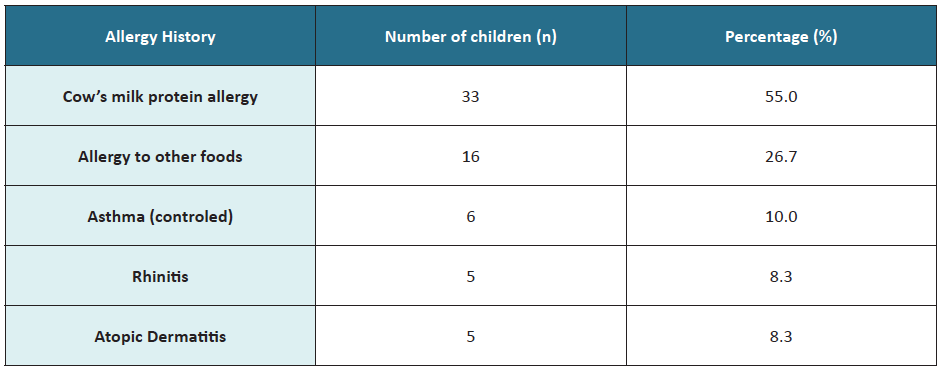
Regarding clinical history, 33 children (55%) had cow’s milk protein allergy, and 16 (27%) allergy to other foods (Table 1).
SPT was performed in 46.7% of children, with most being positive for egg white (Table 2). Most children (95%) were tested for egg yolk- and egg white-specific IgE, being positive for both in most cases. Specific IgE for ovalbumin and ovomucoid was registered in 36 and 33 children, respectively, with most cases positive for ovomucoid. Of the 33 children who performed OFC, 29 were positive.
After the first MMR vaccine dose, three cases of local cutaneous reaction were identified, including pruritic erythematous maculopapular skin rash, and one case of late systemic reaction with periorbital angioedema and urticaria in a child with house dust mite allergy and atopic eczema. The previous allergy study in these children is depicted in Table 3.
Table 2 Skin prick test, specific IgE, and OFC test results
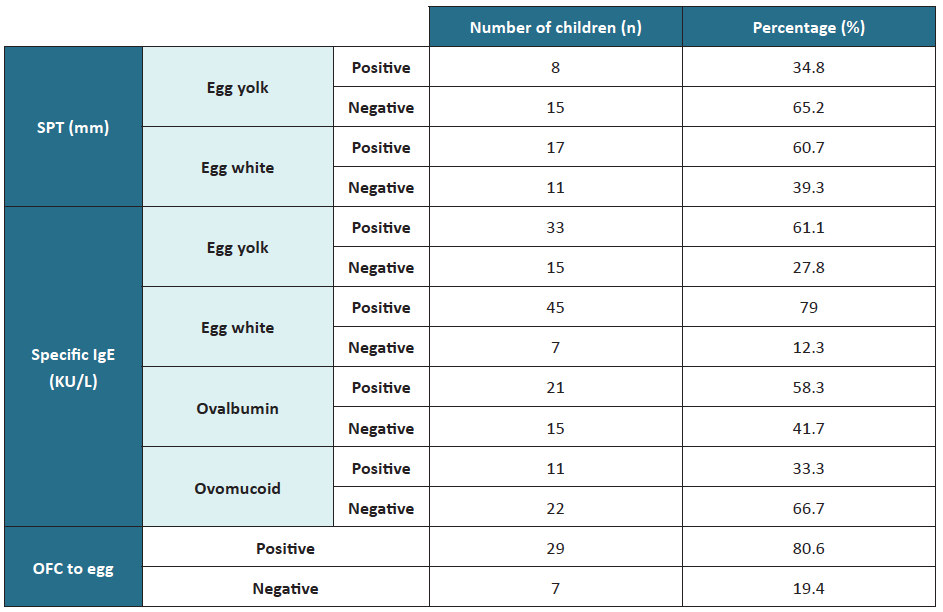
IgE, immunoglobulin E; OFC, oral food challenge; SPT, skin prick test
Table 3 Characterization of patients developing reactions after MMR vaccination
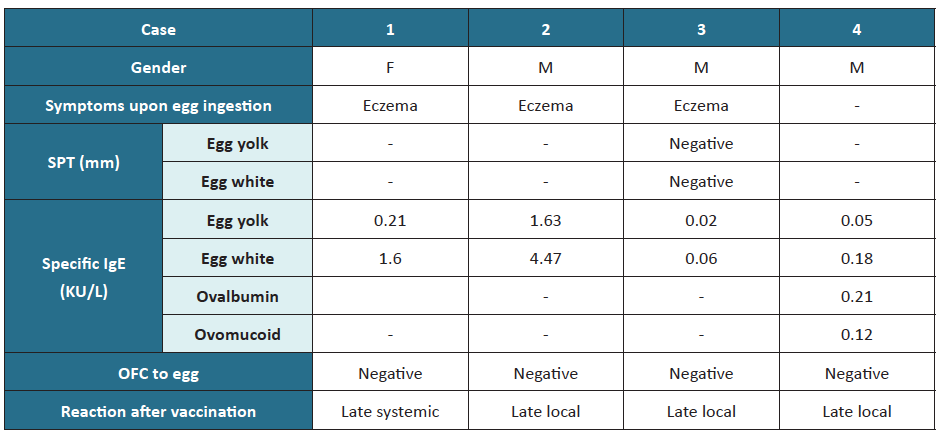
F, female; IgE, immunoglobulin E; M, male; MMR, measles, mumps, and rubella; OFC, oral food challenge; SPT, skin prick test
In children with egg anaphylaxis, SPT was positive for egg white and egg yolk; total IgE was elevated, and specific IgE was positive for ovomucoid, with no record of specific IgE for egg yolk, egg white, or ovalbumin. However, these children showed no allergic reactions to MMR immunization.
Group A included 42 children who had received the first MMR vaccine dose. In these children, the MMR vaccine had been administered with a mean delay of 2.38 months (standard deviation ± 3.0). Of note, in 2012 the Portuguese National Vaccination Plan guidelines were updated, anticipating the first MMR vaccine dose from the age of 15 to 12 months.
Group B included 18 children who received the second immunization dose under the recommended vaccination period, except for two children who were six years old at the time of vaccination. Only one case of a local adverse reaction to a previous MMR vaccine dose was reported in this group.
Discussion
Many studies have shown the safety of MMR immunization in children with or without documented egg allergy.1,2,5-7,9,12-14 Severe adverse reactions to MMR vaccination are very rare, with most reactions being local and transitory and generally occurring due to vaccination adjuvants.
Of the 60 children receiving MMR vaccines in this study, three developed mild local adverse reactions and one developed a late systemic reaction. According to the World Health Organization, local reactions are common and include pain, redness, and/or swelling at the injection site.15 These reactions are caused by non-specific inflammation attributed to the injection itself and to the inoculation of foreign materials and do not constitute a contraindication to immunization.15,16 Delayed urticaria, skin rash, and/or angioedema may occur a few hours after immunization, with immune system activation potentially due to nonspecific degranulation of mast cells.17
In this study, the patient with a previous history of egg anaphylaxis had no allergic reactions after vaccination. This finding is consistent with other studies showing that almost all children, including those with severe egg allergy, tolerate MMR immunization.18-20
In several European countries, MMR vaccination in egg-allergic patients is not a concern anymore. Several studies indicate that these vaccines may contain traces of egg protein in picogram levels, which do not represent a sufficient quantity to cause an allergic reaction.1-3,7,8,10,11,18,21,22 Furthermore, additional studies demonstrate a substantial decrease of anaphylactic reactions since vaccines have stopped including gelatin in their constituents.4,9,23-24 Therefore, MMR vaccination is recommended in all eligible children, independent of egg allergy.3
Although egg allergy is not a contraindication for MMR vaccination, there are still some concerns in the medical community when it comes to MMR immunization in egg-allergic patients, which can lead to delays in the vaccination schedule.2,4,11 In line with this, a mean delay of 2.4 months was observed in the present study in the vaccination calendar of children in Group A.
The fact that this study included a reduced sample size represents a limitation and precludes generalizing the results obtained. Moreover, the retrospective nature of the analysis does not allow to determine the time between hospital referral and MMR immunization. Still, the results retrieved indicate that a history of egg allergy is not a contraindication for MMR vaccine administration. Suspicion of egg allergy should warrant prompt referral to the immunoallergologist, but should not delay MMR immunization.12
The British Society for Allergy and Clinical Immunology (BSACI) and the European Academy of Allergy and Clinical Immunology (EAACI) position paper on vaccination and allergy recommend that infants and children with egg allergy should be vaccinated in the primary care setting as a routine procedure.16,18-25 Further allergic investigations are not recommended before vaccine administration. However, in the present study, many children had performed SPT to egg, specific IgE, or OFC for purposes of diagnosis and treatment of egg allergy itself.
The Portuguese Directorate-General of Health (DGS) published in 2012 a recommendation for the administration of MMR vaccine in all eligible children, independent of previous egg ingestion. Children with egg allergy should receive MMR vaccination in regular vaccination centers without requiring hospital referral despite allergy severity. The guidelines recommend MMR vaccine administration in hospital setting for patients with a history of anaphylaxis due to egg allergy, previous anaphylactic reaction to MMR vaccine or one of its constituents, uncontrolled asthma with documented egg allergy, or uncontrolled asthma with allergy to a previous MMR vaccine dose.26
MMR immunization in patients with suspected or documented egg allergy is still a major reason for hospital referral. However, in this study and according to DGS recommendations, only five cases had true indication for hospital referral. This burden of hospital referrals may lead to unnecessary delays in the vaccination schedule and should be avoided.