Introduction
In the first few months after the outbreak was reported in Wuhan, China, the coronavirus disease (COVID-19) was mostly assumed as asymptomatic or mild in children, with few pediatric hospitalizations and practically no mortality.1 Reports of COVID-19 related severe complications in children were firstly described in April 2020 when predominantly healthy children were hospitalized with cardiogenic shock or Kawasaki disease-like presentations temporally associated with COVID-19 infection.2
The COVID-19 pandemic has represented a massive strain to the health system worldwide. Its severe presentation consists of hyperinflammation with multisystem affection, cytokine storm and elevated myocardial injury markers. Cardiac involvement occurs in 20% to 30% of hospitalized adult patients, and although children have been relatively spared, an increasing number of critically ill children with multisystem inflammatory syndrome (MIS-C, multisystem inflammatory syndrome in children) has been reported.3
The clinical and laboratory findings of COVID-19 in pediatric age are highly variable. The spectrum ranges from asymptomatic infection to acute severe conditions, such as pediatric severe acute respiratory syndrome (SARS) and MIS-C. A small cohort seems to have long-term persistent symptoms that mostly affect their quality of life.4 Symptoms of long COVID-19 in adults have been described and include fatigue, headache, dyspnoea, cognitive impairment, depression, skin rashes and gastrointestinal complaints, but are scarcely described in children.5
Given the substantial impact of COVID-19 in global health care, a race for vaccines has started as soon as the first sequencing data of the virus became available in January 2020. This was even before the disease was declared a global pandemic on March 11, 2020.6 After its approval for general population use in late 2020, increasing evidence for myocarditis and myopericarditis as rare complications of COVID-19 mRNA vaccinations has been surging, especially in young adult and adolescent males.7
Effects of COVID-19 Infection on Children’s Heart
The number of children affected by COVID-19 is lower than the adult population and tend to have milder symptoms, less morbidity and lower mortality.8 Pediatric patients account for ~17,6% of all known COVID-19 infections and mortality rates in this age are as low as 0.1%.9
Children severely affected can present with acute COVID-19 disease or MIS-C symptoms, the latter considered an infection-induced autoinflammatory disease since it usually occurs 3-6 weeks after COVID-19 virus contact and most patients have positive IgG antibody responses.2,10,11 Patients with severe acute COVID-19 disease generally have respiratory involvement with or without multiorgan failure that can affect the heart, whilst MIS-C affected children mostly present with cardiorespiratory involvement that can present with shock.2
A racial disparity has been associated with COVID-19 and MIS-C: Hispanic children and black children have higher rates of infection and more severe disease than non-Hispanic white children and Asians.9,12 There is an estimated rate of 1 MIS-C case per 3164 cases of COVID-19 in children.9
Acute COVID-19 Infection
Most children and adolescents have mild acute COVID-19 disease, 8-19% being completely asymptomatic. The most frequent symptoms, when present, are fever and cough or gastrointestinal complaints. Rarely other severe manifestations have been described, namely encephalitis, seizures, stroke and thromboembolic events (deep vein thrombosis and pulmonary embolism).
Most non-hospitalized children with COVID-19 will not require any specific treatment. The presence of ≥1 comorbid conditions, including cardiac disease, neurologic disorders, prematurity (in young infants), diabetes, obesity (particularly severe obesity), chronic lung disease, feeding tube dependence, and immunocompromised status as well as age (<1 year and 10-14 years) and non-White race/ethnicity is associated with severe disease.9)
Cardiovascular manifestations, unlike in adulthood, are uncommon in pediatric age and may comprehend heart failure, cardiogenic shock, myocarditis, pericarditis, arrhythmia, pulmonary embolism, ST elevation myocardial infarction.
The absolute risk of myocarditis in COVID-19 is low (<0.15%) and, when compared with other causes of myocarditis, children with COVID-19 tend to have a more variable clinical presentation, higher C-reactive protein levels, less need for inotropic support and a shorter time for LV systolic function recovery.11,13
Arrhythmias are usually non sustained and self-limited and encompass ventricular tachycardia, atrial tachycardia and first-degree atrioventricular block. Generally, there is no need for treatment but prophylactic antiarrhythmics have been used in some cases.
Myocardial involvement is associated with elevation of troponin, electrocardiographic and echocardiographic abnormalities and delayed gadolinium enhancement on cardiac magnetic resonance.9
Management of patients
The management of hospitalized children are based largely on adult safety and efficacy data from clinical trials, the child’s risk of disease progression, and expert opinion. However, in young children presenting clinical manifestations common to other respiratory virus (e.g. bronchiolitis, croup) clinical judgement is fundamental to proper care. Adult data are most applicable to older children with severe COVID-19 and especially lower respiratory tract disease.
Supportive care is the basis of management of patients with severe or critical COVID-19 (e.g. respiratory support, including supplemental oxygen and ventilator support, fluid and electrolyte support, interventions to reduce the risk of venous thromboembolism). Treatment should consider the patient’s condition and appropriate monitoring. Glucocorticoids, COVID-19 antiviral therapy and immunomodulatory therapy are used according to the severity of the disease and in selected patients.14-16
Death may occur in most severe cases with myocardial involvement in the form of sudden death or death after intensive medical care and supportive therapy, including extracorporeal membrane oxygenation (ECMO).17)
Feldstein et al analysed the characteristics and outcomes of United States children and adolescents with MIS-C (n=539, 48%) and severe acute COVID-19 (n=577, 52%). Patients with MIS-C needed more vasopressor use (45.3% vs 8.7%) and ECMO (3.3% vs 1.4%). Mortality rate was 1.9% in the first group and 1.4% in the second.17,18
Follow-Up
In our Centre, timing and follow-up of children and adolescents recovering from COVID-19 follows the guidance of the American Academy of Pediatrics (AAP), with surveillance of symptoms by telehealth or in-person visit with specialist according to the clinical status of the patient and are referred to Pediatric Cardiology when there is suspicion of cardiovascular disease (eg. myocarditis, MIS-C).19
Return to play and physical activity will depend on the age of the child, severity of the disease and clinical findings at assessment and should always progress gradually. Children 12 years or older after a moderate to severe disease require a full evaluation before engaging in sports and should be monitored closely.19)
Magnetic resonance studies in young athletes have shown high prevalence of late gadolinium hyperenhancement and abnormal T1/T2 values, even in the setting of previous mild COVID-19 disease, suggesting that the risk stratification for return to competitive activity is yet to be determined and should be addressed very carefully.20
Multisystem Inflammatory Syndrome in Children
Up to 80% of children with MIS-C have cardiac involvement (left ventricular (LV) systolic dysfunction/myocarditis, coronary artery aneurysms [CAA], conduction abnormalities and arrhythmias) and many require intensive care management.11
The major presenting symptoms of MIS-C in a large systematic review by Yasuhara et al (n=917 patients, aged 8.4 to 10.1 years, 56.8% male) included fever (99%), gastrointestinal symptoms (87%, vomiting or diarrhea), abdominal pain (70%), rash (59%), nonpurulent conjunctivitis (57%), oral mucosal changes (42%), neurological symptoms (36%) and peripheral extremity changes (32.9%).21 LV systolic dysfunction defined as LV ejection fraction< 50% or myocarditis occurred in 55.1%, CAA or dilatation in 21.7% and pericardial effusion in 31.7% of the children. Inflammatory and cardiac markers (B-type natriuretic peptide, N-terminal proB-type natriuretic peptide and troponin) were elevated. Admission in Intensive Care Unit was required in 79.1% of the cases. The most common therapies were intravenous immunoglobulin (81.9%9, aspirin (67.3%), corticosteroids (63.6%), inotropes (62.9%), anticoagulation (56.5%); several antiviral agents and anti-inflammatory biologics (54.8%). Non-invasive ventilation and mechanical ventilation were needed in respectively 24.6% and 33% of cases. The rate of ECMO was 6.3%. At discharge recovery of LV ejection fraction (LVEJ>60%) was observed in 55.1% of patients. Mortality rate was 1.9%.21
Management of patients
To better understand MIS-C, clinicians should be aware of the similarities with other autoinflammatory diseases, such as Kawasaki Disease (KD), Toxic Shock Syndrome and Hemophagocytic Syndrome.10 In fact, the first cases of MIS-C were described as children hospitalized with post COVID-19 cardiogenic shock or KD-like presentation.2 Additionally, most of the immunomodulatory therapy, like intravenous immunoglobulin (IVIG) and steroids, has shown to be effective in the treatment of MIS-C.10) In severe cases, the use of adequate inotropic support and anticoagulation has been advocated with positive outcome, as stated above.11
Although several symptoms overlap between KD and MIS-C, like prolonged fever and mucocutaneous lymph node symptoms, the latter presents unique features such as prone to later onset, gastrointestinal symptoms and tendency to left ventricular (LV) systolic dysfunction.22 Table 1 compares the main clinical differences between MIS-C and KD.
Table 1 Differential diagnosis between MIS-C and KD.
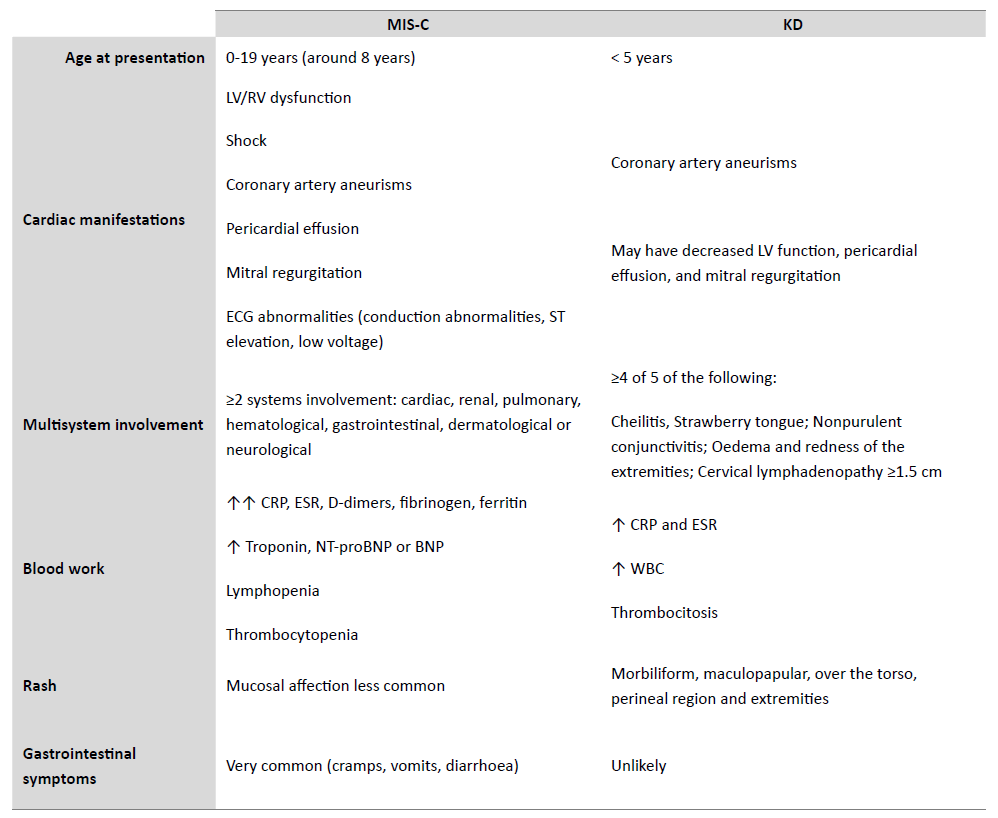
MIS-C Multisystem Inflammatory Syndrome in Children; KD Kawasaki Disease; CRP C-reactive Protein; ESR Erythrocyte Sedimentation Rate; LV Left Ventricle, RV Right Ventricle; WBC White Blood Cell
There are three known mechanisms that can be involved in myocardial injury: the two classic mechanisms of direct viral replication injury versus cardiac necrosis secondary to inflammatory infiltrates. The third process consists in microvascular injury with microthrombi formation in small vessels and significant rise in d-dimers, thus the indication for antiplatelet and anticoagulation therapy in severe cases that an increasing number of specialists advocates.3
The early outcome of MIS-C, especially in children treated with immunomodulators, is generally favourable with resolution of inflammation and cardiovascular abnormalities within one to four weeks of diagnosis.9 Most cases of LV function impairment in MIS-C completely resolve in 1-2 weeks, suggesting that myocardial injury might be a result of acute stress and severe systemic inflammation, rather than ischemia or direct virus-mediated myocardial damage.2 CAA are more common in male patients and in those with mucocutaneous lymph node symptoms and have an estimated prevalence of 13-26%. CAA are usually small, regressing completely within 3-5 weeks in the population treated with IVIG.2,11 This may be evidence that vasodilatation in the setting of high inflammation state is responsible for CAA occurrence, rather than vascular wall destruction as seen in KD.
Prolongation of all ECG intervals has been observed in patients with MIS-C, particularly the PR interval. First-degree atrioventricular block may occur in up to 25% of patients and a higher degree A-V block has been described in 7%, particularly in patients with LV dysfunction. Cases of severe sinus bradycardia, ST segment changes and tachyarrhythmia have also been described.11
Prognosis and follow-up
MIS-C is an uncommon but potentially life-threatening complication of COVID-19. The mortality rate of MIS-C is 1.4% to 1.9% and compared with children who died of acute COVID-19 infection, most of the fatalities were in previously healthy individuals without comorbidities. Long term follow-up data are limited, but prognosis seems positive as most children present a full recovery, with a normal LV ejection fraction within several weeks. However, Matsubara et al found persistent abnormalities in strain and diastolic function in patients with MIS-C and normal EF, which suggests that subclinical myocardial injury may persist.18,23 These findings outline the need of a longitudinal cardiovascular and imaging follow-up of these patients to assess LV systolic and diastolic function and better understand the mechanisms of myocardial involvement in MIS-C.
The return to normal physical activity depends on the clinical status and disease severity. In case of myocarditis, restriction from exercise for three to six months is recommended.9
MIS-C and Acute Rheumatic Fever
As an infection induced autoinflammatory disease, MIS-C shares key characteristics with acute rheumatic fever (ARF), a multiorgan affecting disease that can also strike the heart.24 Some authors even consider MIS-C the 21st century ARF. The similarities between the two include the associated pathogen, the mild course of infection preceding the disease in few weeks and the occurrence in childhood.10 ARF diagnosis sits in Jones criteria that requires proof of group A Streptococcus (GAS) preceding infection by means of positive throat culture, a positive rapid carbohydrate test or a rising titre of streptococcal antibodies (anti-streptolysin O, anti-DNase B).10,25 In turn, the criteria to diagnose MIS-C includes known exposure to COVID-19 that can be proved by prior RT-PCR or antigen test or serologic analysis.10
Several exposures to GAS and genetic predisposition are thought to be vital for the immune response that ultimately originates ARF. This immune response leads to the production of antibodies able to cross-react with antigenic epitopes shared between host and bacteria, particularly laminin, a protein present in extracellular matrix that surrounds heart cells and in the valves. The persistence of COVID-19 virus on the mucosa or the multiple exposures to COVID-19 parents or previous coronavirus infections in infancy are hypothesized mechanisms for primming of the immune system to produce antibodies with molecular mimicry properties that are thought to cause MIS-C (24). Figure 1 illustrates the hypothesized overlaps of the pathophysiology between MIS-C and ARF.
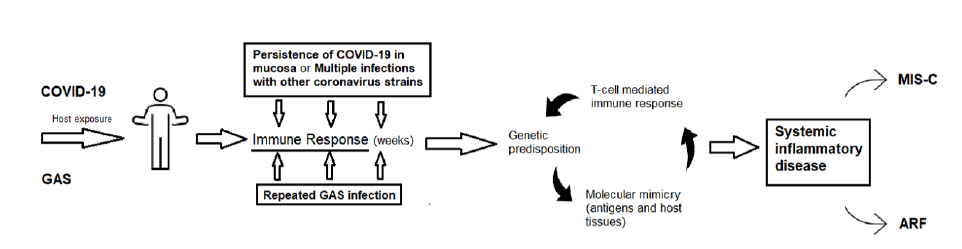
Figure 1 Common theoretical pathophysiology between MIS-C and ARF. ARF Acute Rheumatic Fever; GAS Group A Streptococcus; MIS-C Multisystem Inflammatory Syndrome in Children. Adapted from: Pediatric Inflammatory Multisystem Syndrome Temporally Related With SARS-CoV-2: Immunological Similarities with Acute Rheumatic Fever and Toxic Shock Syndrome (Buonsenso et al.)24
Myocarditis following COVID-19 mRNA Vaccination
Myocarditis after mRNA vaccines for COVID-19 has been described in pediatric age, especially in male adolescents and young adults particularly after the second dose.7 This outcome has previously been seen, though rarely, in vaccines for other pathogens, such as smallpox and Influenza.11,26 The Center for Disease Control and Prevention (United States) stated that myocarditis/pericarditis rates are around 12.6 cases per million doses of second-dose mRNA vaccine among individuals 12 to 39 years of age. Multiple mechanisms have been proposed (molecular mimicry between the spike protein and self-antigens, immune response to mRNA, activation of immunologic pathways and dysregulated cytokine expression…), but none explain the male preponderance.7 The course of the disease is usually benign, resolving in a few days after diagnosis, with or without treatment.7,26 Thus, the beneficial-risk assessment of the vaccine administration shows a favourable balance for all age and sex groups.7
The “Long COVID” in Pediatric Age
A large cohort study in Jin Yin-tan Hospital in Wuhan (China) of early 2020 included 1733 adults post COVID-19 infections and stated that 76% had persistent symptoms 6 months after initial diagnosis. The most common complaints were muscle weakness (63%), sleep difficulties (26%) and depression or anxiety symptoms (23%) and they were positively correlated with severity of the infection and length of hospital stay.26 Increasing evidence of long COVID-19 has come to light, but data in pediatric populations is still limited but apparently younger children have fewer persistent symptoms than older children and adults.27
One of the first large series study done in children took place in Fondazione Policlinico Universitario A. Gemelli IRCCS (Rome, Italy).28 One hundred and twenty-nine children with previous COVID-19 infection with median age of 11.4 years old were enrolled and assessed for persistent symptoms after a period on average of 162.5 ± 113.7 days after initial infection. Insomnia (18.6%), respiratory symptoms including pain and chest tightness (14.7%), nasal congestion (12.4%), fatigue (10.8%), muscle (10.1%) and joint pain (6.9%), and concentration difficulties (10.1%) were the most frequently reported symptoms, described in both children with symptomatic and asymptomatic acute COVID-19.
Another prospective study by Ashkenazi-Hoffnung et al assessed 90 children with mean age 12 ± 5 years at a median of 112 days after COVID-19 diagnosis.28 Despite a mild acute disease and lack of background illness in most children, nearly 60% had symptoms with functional impairment and school absence at 1-7 months after the onset of infection.5,19) These symptoms included: fatigue (71%), dyspnoea (50%), myalgia (46%), sleep disturbances (33%), chest pain (31%), paresthesia (29%), headache (29%), hair loss (27%), anosmia-ageusia or parosmia/euosmia (26%), gastrointestinal symptoms (20%), dizziness (19%), weight loss of >5% of body weight (19%), memory impairment (18%), vasomotor complaints (14%), arthralgia (14%), tremor (13%), cough (10%), palpitations (9%), difficulty in concentration (9%), tic exacerbation (2%) and tinnitus (1%).
A sub cohort of 60 patients who underwent pulmonary function tests due to cardiorespiratory symptoms had abnormal findings, namely a mild obstructive pattern (low values of forced expiratory volume in the first second and air trapping on lung volume evaluation).28 Half of these patients responded to bronchodilators. Sixty-three patients underwent cardiac investigation: none of them had abnormalities on echocardiogram and two patient displayed abnormal ECG.
Conclusions
Low rates of heart involvement in acute COVID-19 infections have been reported and it usually follows severe multisystemic affection and children with comorbidities. MIS-C, in turn, is an autoinflammatory syndrome that takes place around four weeks after COVID-19 infection in children and, although rare, it has a high probability of heart abnormalities. Current treatment strategies mostly rellie on treating the hyperinflammation and have proven effective at resolving many of these cardiac findings, but there is still room for improvement. Early diagnosis and treatment may change the course of severe disease and the role of the Cardiologist assessment is key.
It is essential to do a differential diagnosis between other know autoinflammatory states to correctly assess and treat, although most of them have common treatment strategies.
Despite the potential for severeness and complications, COVID-19 and MIS-C mortality rates are low and short-term outcomes are favourable. Long-term follow-up for chronic complications and persistent symptoms is crucial. Data from recent studies confirm the morbidity associated with long COVID-19 in children and highlight the importance of multidisciplinary pediatric clinics for evaluation and treatment, although no specific heart condition has been described with long COVID-19.
There is still a long way to go to have a better insight of the full spectrum of COVID-19 related manifestations in children. Further studies and research are vital to understanding the mechanisms and natural history of the disease, diagnostic features, risk stratification, ideal management and long-term outcomes. Specific gaps such as the development of antiviral drugs with proven safety and effectiveness in children, biochemical diagnostic markers, severity predictors and likelihood of long-term affection need to be addressed.
Vaccination is recommended for all age and sex groups, despite rare cases of self-limited myocarditis associated administration.















