Introduction
Acute respiratory infections (ARI) are an important cause of morbidity and mortality in children.1 Lower respiratory tract infections, which include tracheitis, bronchitis, bronchiolitis, and pneumonia, are a common cause of hospital admission in pediatric age.1,2 Incidence and hospitalization rates are particularly high under the age of two years, subsequently decreasing with age.3 No difference seems to exist in sex distribution.4 Viruses are the etiological agents most frequently involved, with more than 200 respiratory viruses capable of causing ARI.3,4 Associated signs and symptoms are similar among etiological agents and include fever, sore throat, cough, rhinorrhea, nasal congestion, sputum, shortness of breath, lung auscultation abnormalities, tachypnea, and chest pain.4,5 Despite seldom affecting the treatment regimen (except in the case of influenza viruses), the etiological diagnosis may have an important role in decreasing unnecessary antibiotic use, as well as reinforcing isolation measures to prevent nosocomial transmission.6 Viral pathogens can be identified by viral culture, direct immunofluorescence assays, rapid antigen tests, and/or nucleic acid tests, the latter being associated with higher sensitivity.4,5 As these techniques become more promptly available, detection rates also rise, varying from 50-75%, with higher figures when only considering epidemic seasons.3,4 However, viral detection does not necessarily correlate to viral infection and may represent asymptomatic persistence instead, which can go up to six weeks.7
The most frequently identified agent is the respiratory syncytial virus (RSV), either isolated or with other agents in the setting of coinfection, with a higher prevalence in winter.2 Influenza and parainfluenza viruses are also commonly detected.8 However, while the incidence of RSV decreases with age, the contrary is observed for influenza viruses.4 Some viruses, such as enteroviruses, adenoviruses, and rhinoviruses, are equally prevalent throughout the year.2 Parainfluenza viruses are also frequent, particularly in the United States. Metapneumoviruses appear to be more frequent in Europe and under the age of 12 months.4 Viral coinfection rates vary from 10-44%.2,4 Coinfection is significantly more prevalent in winter and in children under the age of two years, possibly due to an immature immune system.2,4 Few studies have considered children with and without comorbidities separately.9
The association between infection with multiple respiratory viruses and disease severity in children has not been properly established.3 Whereas some studies report an increase in illness severity, namely in hospitalization rates, hospital length of stay, Intensive Care Unit admission, need for mechanical ventilation, and higher mortality, systematic reviews and meta-analyses, including the one by Lim et al. that included 19 studies, showed no statistically significant differences in the outcomes of children with infection by one versus multiple viruses.3,9,10 This result is consistent with those found by Scotta and colleagues.3
The aim of this study was to characterize children with viral respiratory tract infections admitted to a Pediatric Department and investigate whether viral respiratory coinfection is associated with a more severe clinical course.
Materials and methods
This was a single-center cross-sectional study of children with ARI admitted to the Pediatric Department of a level II hospital in Portugal who underwent immunofluorescence analysis for respiratory viruses on nasopharyngeal aspirates. Children over 28 days and under five years were included. As a ten-virus panel was available at the study center, a thirteen-month time period was considered (December 2017−December 2018). The panel included Adenovirus (AdenoV), Bocavirus (BocaV), Coronavirus OC43 (CoronaV), Influenza A (InfA), Influenza B (InfB), Metapneumovirus (MPV), Parainfluenzae 1 (PI 1), Parainfluenzae 2 (PI 2), Parainfluenzae 3 (PI 3), and RSV. For the analysis of monthly distribution, only the year 2018 was considered.
Patients with at least one virus identified were included, and the following data were retrieved from their clinical records: sex; age; viruses identified; risk factors for lower respiratory infection (preterm birth, low birth weight, exposure to second-hand smoke, absence of breastfeeding, congenital cardiovascular defects, bronchopulmonary dysplasia, and immunodeficiency); clinical presentation (fever, cough, coryza, sputum, signs of respiratory distress, altered pulmonary auscultation); use of supplemental oxygen; maximum oxygen supply flow; oxygen delivery device required; need for bronchodilators; need for inhaled, oral, and/or intravenous corticosteroids; hospital length of stay, transferal to a tertiary care unit; and death.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS®) version 26 software by IBM, and p-values <0.05 were considered statistically significant.
Age, maximum oxygen flow, duration of supplemental oxygen, and hospital length of stay were reported as median and interquartile range (IQR). The remaining variables were categorical and reported as absolute numbers and frequencies.
Participants were compared according to the identification of one versus two viruses in virologic test results. The Mann-Whitney U test was used to compare age, maximum oxygen flow, duration of supplemental oxygen, and hospitalization days between groups. Pearson’s Chi-Square or Fisher’s Exact test (when assumptions for the first were not verified) were used to compare categorical variables.
Results
Characteristics of the study cohort
Among 434 patients included in the considered study period, 224 had positive results in immunofluorescence analysis. Of these, 124 (55.4%) were male. Participants were aged between one month and five years, with a median of 0.50 years (IQR 0.17-1.00) and 89.7% under the age of 12 months. At least one risk factor was identified in 57.1% of patients (n=128): 17.4% had a preterm delivery, 15.2% had low birth weight, 39.3% had been exposed to second-hand smoke, 11.6% had not been breastfed, 3.1% had a congenital cardiovascular defect, and 1.8% had bronchopulmonary dysplasia. None had immunodeficiency. The most common clinical manifestations in this cohort were cough, altered pulmonary auscultation, and signs of respiratory distress (SRD), such as tachypnea, retractions, cyanosis, nasal flaring, and wheezing (Table 1).
Viral detection
In total, 434 nasopharyngeal samples of children under the age of five years were processed. At least one virus was identified in 51.6% (n=224). The coinfection rate was 5.8% (n=13). No patient tested positive for more than two viruses simultaneously. Table 2 depicts the prevalence of each virus and coinfections identified. RSV and adenoviruses were the most commonly detected viruses, both overall and in the age groups under 24 months. Table 3 shows the viral distribution according to age group. The most common association was between RSV and Coronavirus OC43, corresponding to 38.5% of cases. Sixty-nine percent of coinfections included RSV.
Seasonality
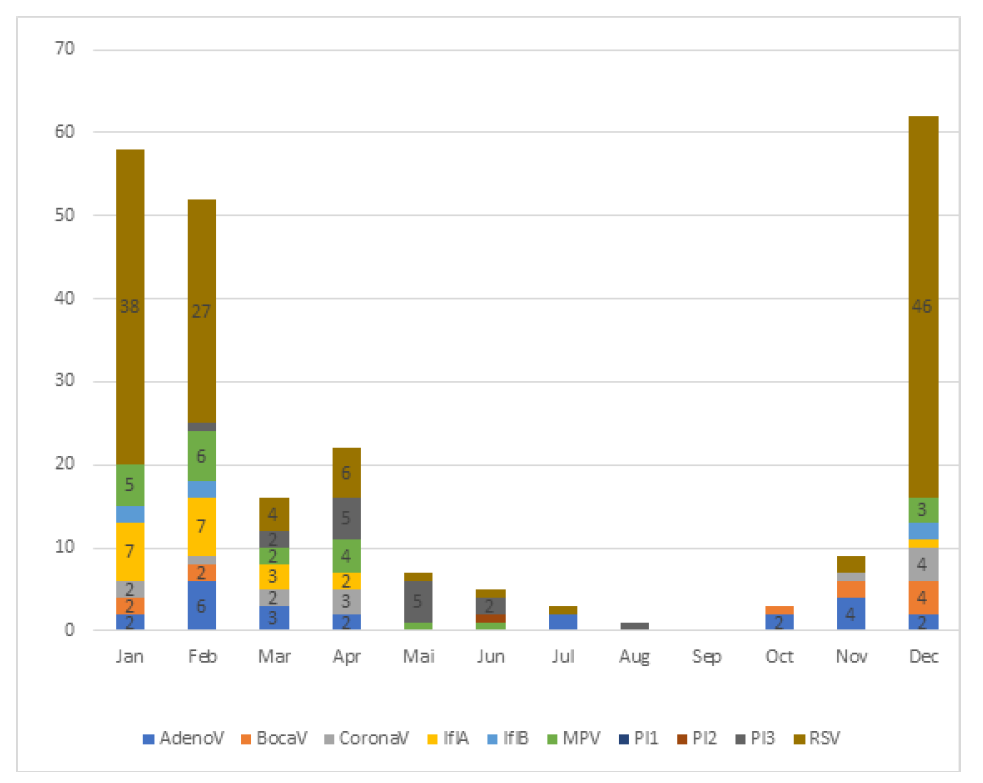
Figure 1 shows the monthly distribution of positive cases, as well as the prevalence of each virus per month. All viruses were more prevalent between November and February, except for Parainfluenzae 3 virus, which was more common in April and May, and Parainfluenzae 2 virus, which was only identified in June. January was the month with the highest prevalence of positive results. Coinfections were more frequent in April.
Clinical Severity
Forty-four percent of patients required supplemental oxygen. In 82.7%, a nasal catheter was sufficient as interface, 5.1% required high-flow oxygen supply, and 8.2% required a non-rebreather mask.
Five percent (n=12) of cases were transferred to a tertiary care unit, although none had coinfection identified. The median oxygen flow provided was 1 L/min (IQR 1-2), the median duration of oxygen supply was three days (IQR 2-5), and the median hospitalization time was four days (IQR 3-6). Regarding treatment, 178 (79.5%) patients were prescribed inhaled bronchodilators, 40 (17.9%) inhaled corticosteroids, 80 (35.7%) oral corticosteroids, and 86 (38.4%) intravenous corticosteroids.
Group comparisons
Sex distribution (p=0.910) and median age (U=1223.00, p=0.509) were similar between groups. All patients presenting with coinfection were under 12 months. The percentage of patients with at least one risk factor identified was higher in the coinfection than in the monoinfection group, although this difference was not statistically significant (69.2% vs. 56.4%; p=0.364.
Coinfected patients only required nasal catheter as oxygen delivery system. No statistically significant differences were found between groups regarding maximum oxygen flow (U=184.550, p=0.261) or days of oxygen supply (U=180.000, p=0.341). The percentage of patients submitted to intravenous corticosteroids was similar between groups, with the same being true for hospital length of stay (excluding transferred patients; U=1362.000, p=0.966).
Table 1 Comparison between single infection and coinfection groups regarding sex, age, presence of at least one risk factor, clinical manifestations and clinical severity.
| Single infection n= 211 | Coinfection n= 13 | Total n= 224 | p value | |
| Sex | ||||
| Feminine | 94 (44.5%) | 6 (46.2%) | 100 (44.6%) | 0.910* |
| Masculine | 117 (55.4%) | 7 (53.2%) | 124 (55.5%) | 0.910* |
| Age (years) | 0.50 (0.17-1.00) | 0.50 (0.29-0.84) | 0.50 (0.17-1.00) | 0.509‡ |
| Age group | ||||
| <12 mo | 188 (89.1%) | 13 (100%) | 201 (89.7%) | 0.371† |
| 12-24 mo | 13 (6.2%) | - | 13 (5.8%) | 1.000† |
| 24-36 mo | 4 (1.9%) | - | 4 (1.8%) | 1,000† |
| >36 mo | 6 (2.8%) | - | 6 (2.7%) | 1,000† |
| ≥ 1 risk factor | 119 (56.4%) | 9 (69.2%) | 128 (57.1%) | 0.364* |
| Clinical manifestations | ||||
| Fever | 129 (61.1%) | 8 (62.5%) | 137 (61.2%) | 0.977* |
| Cough | 187 (88.6%) | 12 (92.3%) | 199 (88.8%) | 1.000† |
| Rhinorrhea | 87 (41.2%) | 4 (30.8%) | 91 (40.6%) | 0.456* |
| Nasal obstruction | 91 (43.1%) | 5 (38.5%) | 96 (42.9%) | 0.741* |
| Sputum | 109 (51.7%) | 9 (69.2%) | 118 (83.9%) | 0.218* |
| SDR | 155 (73.5%) | 9 (69.2%) | 164 (73.2%) | 0.751† |
| Altered pulmonary auscultation | 166 (78.7%) | 9 (69.2%) | 175 (78.1%) | 0.480† |
| Clinical severity | ||||
| Length of hospital stay (days) | 4.0 (3.0-6.0) | 4.0 (3.0-7.0) | 4.0 (3.0-6.0) | 0.966‡ |
| Need for supplemental O2 | 92 (43.6%) | 6 (46.2%) | 98 (43.8%) | 0.857* |
| Maximum Oxygen Flow (L/min) | 1.0 (1.0-2.0) | 1.00 (0.88-1.25) | 1.0 (1.0-2.0) | 0.261‡ |
| Supplemental O2 duration (days) | 3.5 (2.0-5.0) | 2.50 (1.75-4.75) | 3.0 (2.0-5.0) | 0,341‡ |
| Transferal to tertiary care | 12 (5.7%) | - | 12 (5.4%) | 1,000† |
| Inhaled Bronchodilators | 169 (80.1%) | 9 (69.2%) | 178 (79.5%) | 0.311† |
| Inhaled corticosteroids | 39 (18.5%) | 1 (7.7%) | 40 (17.9%) | 0.473† |
| Oral corticosteroids | 73 (34.6%) | 7 (5.4%) | 80 (35.7%) | 0.232† |
| Intravenous corticosteroids | 81 (38.4%) | 5 (38.5%) | 86 (38.4%) | 1.000† |
Categorical variables are presented as count (percentage) and continuous variables as median (interquartile range). *p-value according to Chi-square test. †p-value according to Fisher’s Exact test. ‡p-value according to Mann-Whitney test. mo=months.
Table 2 Distribution of viruses identified in single- and coinfections. Numbers represent counts
| AdenoV | BocaV | CoronaV | InfA | InfB | MPV | PI 1 | PI 2 | PI 3 | RSV | |
| AdenoV | 18 | 1 | 2 | 2 | ||||||
| BocaV | 1 | 9 | 1 | |||||||
| CoronaV | 2 | 6 | 5 | |||||||
| InfA | 20 | |||||||||
| InfB | 4 | |||||||||
| MPV | 0 | 1 | ||||||||
| PI 1 | 22 | |||||||||
| PI 2 | - | |||||||||
| PI 3 | 15 | 1 | ||||||||
| RSV | 2 | 1 | 5 | 1 | 1 | 116 | ||||
| Total | 23 | 11 | 13 | 20 | 4 | 1 | 22 | - | 16 | 126 |
Table 3 Viral detection distributed by age group. Numbers represent counts. mo=months
| Age group | ||||
| <12 mo | 12-24 mo | 24-36 mo | >36 mo | |
| AdenoV | 21 | 2 | - | - |
| BocaV | 11 | - | - | - |
| CoronaV | 12 | 1 | - | - |
| InfA | 17 | - | 2 | 1 |
| InfB | 3 | 1 | - | - |
| MPV | 20 | 1 | 1 | - |
| PI 1 | - | - | - | - |
| PI 2 | - | - | - | 1 |
| PI 3 | 15 | - | 1 | - |
| RSV | 114 | 8 | - | 4 |
| Total | 201 | 13 | 4 | 6 |
DISCUSSION
In the present study, at least one virus was identified in 51.6% of cases, and coinfections were identified in 5.8% of these cases. The corresponding prevalence in the literature is 50−75% and 10−44%, respectively, varying according to region, season, year, population characteristics, and detection methods employed.3,4 Since all seasons were considered in this study, the positivity rate found was lower compared to other studies that included only epidemic seasons. Additionally, immunofluorescence assay was the detection method employed here, which has an estimated sensitivity of 80−90%, whereas most studies rely on polymerase chain reaction (PCR) amplification, which offers higher sensitivity rates (95%).11,12
The absence of Rhinovirus in this study’s viral search panel surely contributes to lessening the overall positivity and coinfection rates observed since studies report a high prevalence of this virus, as well as a significant impact on clinical outcomes.13,14
The higher incidence of coinfections in this study compared to that reported by Antunes et al. is likely attributed to the inclusion of Coronavirus OC43 in the analysis since this is the second most prevalent virus in coinfections, after RSV.11 RSV is also the most common virus identified in single infections (≈50% of cases). This is consistent with prior studies.15,16 Conversely, a study in France has reported a high incidence of Metapneumovirus, which was only found in a single case in this study.13 The second most common virus found in this study was Adenovirus, which has a variable prevalence in the literature, even in countries geographically close to Portugal, such as Spain.17,18
A similar distribution of respiratory viruses was found between genders, confirming previous reports disproving a sex-based predisposition to respiratory viral infections. On the other hand, a decrease in the detection rate of respiratory viruses was observed with age, with most infections (89.1%) occurring in the <12-month-old group. This is consistent with previous studies suggesting lower susceptibility to respiratory viral infections in older individuals.18,19 Some studies suggest that coinfection rates are higher in younger children, mostly compared to school-aged children and adolescents, perhaps due to an immature immune system.6 Keeping in mind the small size of the coinfection sample in this study, its findings suggest the absence of a statistically significant difference in coinfection rates according to age in children under five years old.
As expected, the overall viral prevalence was higher in November through February, peaking in January. Parainfluenza 2 and 3 were more frequent in spring. Surprisingly, coinfections were more common in April. This disagrees with reports in the literature of a higher coinfection rate in colder months.18
The most frequent signs and symptoms identified in this patient population were cough, respiratory distress syndrome, and altered pulmonary auscultation, with similar prevalence in both groups. Since RSV is the main etiological agent causing SRD and the most frequent virus in both groups, this may explain why no differences were found in clinical features between groups.11
No statistically significant association was identified between coinfection and increased clinical severity, which is consistent with the most recent meta-analysis on the subject.3 Hospitalization rates were not considered in the present analysis since testing was only performed in inpatients. There was no increased use of intravenous corticosteroids or supplemental oxygen, longer hospital stays, or more frequent transferals to tertiary care facilities in this study population, in contrast with a similar study in Portugal that found increased hospital lengths of stay and use of bronchodilators and oral corticosteroids in children with viral coinfections.18 Other studies reported an increase in patients’ transferal rate to Intensive Care Units (ICU) and identified coinfection as an independent risk factor for ICU transferal.6,13 Although coinfections were slightly more frequent in patients with risk factors for ARI compared with patients without risk factors, the difference was not statistically significant.
Still, the role of codetection remains unclear. A study using molecular assays reported a prevalence of nine percent for the detection of four viruses simultaneously. 19 Findings of this study, using immunofluorescence as detection method and thus with fewer false positives, support the absence of an association between multiple viral detection and clinical severity.
The present study has limitations, such as its small sample size and particularly the small number of coinfections assessed. Therefore, more statistically powered studies are necessary to confirm its findings. On the other hand, extending the present analysis for several years would be relevant to characterize the annual variation in the local prevalence of each virus.
Clarifying the role of viral coinfections in respiratory infections is crucial since the belief of greater clinical severity associated with multiple infections may cause unnecessary anxiety in clinicians and caregivers.
Conclusions
The etiologic diagnosis is important in respiratory infections to minimize nosocomial transmission, begin appropriate antiviral treatment in selected cases, and lower antibiotic misuse.
Consistently with previous studies, this analysis found that RSV is the most important etiological agent both in single and coinfections. A viral coinfection rate of 5.8% was identified, with the most frequent viral association being between RSV and Coronavirus OC43. Viral coinfection is not associated with greater clinical severity, specifically regarding hospital length of stay, intravenous corticosteroid use, higher supplemental oxygen needs, or more frequent ICU transferal. The prevalence of patients with at least one risk factor for respiratory infections was higher in the coinfection group, although this was not statistically significant.
List of abbreviations
AdenoV |
-Adenovirus |
ARI |
-Acute respiratory infection |
BocaV |
-Bocavirus |
CoronaV |
-Coronavirus OC43 |
ICU |
-Intensive Care Unit |
InfA |
-Influenza A virus |
InfB |
-Inflenza B virus |
IRI |
-Infeção respiratória inferior |
MPV |
-Metapneumovirus |
PI1 |
-Parainfluenzae virus 1 |
PI2 |
-Parainfluenzae virus 2 |
PI3 |
-Parainfluenzae virus 3 |
RSV |
-Respiratory syncytial virus |
SDR |
-Signs of respiratory distress |
Authorship
Patrícia Sousa - Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Visualization; Writing - original draft; Writing - review and editing
Nadezda Kochetkova - Data curation; Investigation; Methodology; Resources; Writing - review and editing
Susana Correia de Oliveira - Data curation; Investigation; Methodology; Resources; Writing - review and editing
Paula Mota - Conceptualization; Data curation; Methodology; Resources; Supervision; Writing - review and editing
Ângela Dias - Conceptualization; Methodology; Project Administration; Supervision; Writing - review and editing