Introduction
In recent years, there has been a global increase in the prevalence of immunoglobulin E (IgE)-mediated food allergy, particularly in pediatric patients.1 In Europe, a recent meta-analysis concluded that the self-reported lifetime prevalence of food allergy, in patients under 18 years of age, was 18.7%.2) The same study found that the reported physician-diagnosed prevalence of any food allergy, in the same age group, was 9.3%. Another study estimated that the incidence of documented food allergy in European schoolchildren ranged from 1.4% to 3.8%.3) It is well known among physicians that many parents often misinterpret common signs and symptoms as secondary to food ingestion, which may lead to a misperception of food allergy.4,5 This fact may justify the variability between proven and self-reported prevalence of food allergy mentioned above. In addition, this parental overestimation of food allergy often leads to unnecessary avoidance diets that negatively impact children’s overall well-being.6
The most common food allergens can be classified into eight major groups: cow’s milk, hen’s egg, peanuts, tree nuts, wheat, soy, fish, and shellfish.7) Despite the high variability observed in the prevalence of each food allergy in different geographical locations, in general, cow’s milk and hen’s egg are the most commonly implicated food allergens, particularly in the early years.8 In European school-aged children (6-10 years), peanut and hazelnut have been reported as the most common food allergens.3 However, in Portugal, allergy to fresh fruit, fish, and eggs appear to be the most common food allergies in children aged three to 11 years.9 Lastly, according to a recently published study, fresh fruit, shellfish, and tree nuts were the most commonly reported food allergens in Portuguese adolescents.10
Given its socioeconomic burden, food allergy has become a major health problem worldwide.11) Its proper management requires timely assessment, thorough anamnesis, and testing.12 Skin prick testing and determination of food-specific serum IgE antibodies are known to be sensitive tools for the assessment of a suspected allergy, but the sole presence of allergen sensitization does not necessarily correlate with clinical symptoms upon food ingestion.13 Therefore, it is often necessary to perform an oral food challenge (OFC) to differentiate between a patient who is merely sensitized to an allergen and a patient who is clinically reactive to it.12,13 Placebo-controlled and double-blinded OFCs are considered the gold standard for the accurate diagnosis of food allergy. However, in daily pediatric practice, most tests are performed in an open fashion because it is less costly and time-consuming. OFCs can also be used to assess the resolution of a previously known allergy or to evaluate the amount of food required to trigger symptoms, known as the threshold of responsiveness.12) Nevertheless, OFCs carry risks, as positive reactions can range from mild to potentially life-threatening.12 Therefore, it is fundamental to document and analyze any possible predictors of a positive result.
The aim of this study was to assess the outcomes of the OFCs performed in the Pediatric Department of the study hospital and to assess any possible factors predictive of its results.
Material & methods
A retrospective review of all patients who underwent OFCs from October 2016 to December 2021, at a level II hospital, was performed. Eight OFCs were excluded, all concerning non-IgE mediated allergies.
Tests were performed as open challenges, in an outpatient setting and under medical supervision. Prior to testing, patients were carefully selected by a pediatrician with experience in allergy based on their medical history, skin prick test results, and/or the determination of serum food-specific IgE. Testing was performed at least six weeks after a previous reaction. Children with a recent history of anaphylaxis were not submitted to OFCs.
All OFCs were performed according to the Pediatric Department’s protocol, which was developed according to the recommendations of the PRACTALL consensus report, as well as the consensus previously published by the European Academy of Allergology and Clinical Immunology and the American Academy of Asthma, Allergy & Immunology, regarding OFC standardization.14-16) Prior to testing, all patients and their families were fully informed about the procedure and provided informed consent. If the child had a chronic condition requiring treatment with inhaled or topical steroids, leukotriene antagonists, or inhaled β-agonists, these had to be used at the lowest possible dose (while ensuring adequate disease control). Antihistamines had to be discontinued for a period of five half-lives of the specific agent prior to the OFC. Patients had to be asymptomatic on the day of testing.
For each food tested, the used protocol specified how many milligrams (mg) of food should be consumed to deliver the exact amount of food protein intended for the challenge. Food protein was administered in semi-logarithmic incremental doses (i.e., 3, 10, 30, 100, 300, 1000, and 3000 mg), at 20-minute intervals. For high-protein foods (e.g., fish), for which the maximum cumulative dose might not represent the amount of protein typically consumed in a meal, a final step was performed with an age-appropriate serving of the native food. For foods with low protein content, such as most fruits, the final dose of 3000 mg was not administered. In most cases, foods were offered unprocessed, except for fish, shellfish, cereals, and eggs (the latter were challenged both raw and cooked).
All acute reactions were scored according to the scoring system available in the PRACTALL consensus report.14) This system indicates symptoms and signs that may warrant caution or that may justify stopping the challenge. After the procedure, patients remained under observation for at least two hours. At discharge, they were informed about possible late reactions and were instructed to seek medical attention if experiencing any symptoms. A test was considered negative if there were no reactions during the challenge or within the observation period. A test was considered positive if there were any objectifiable IgE-mediated symptoms or if the patient experienced persistent subjective symptoms consistent with IgE-mediated reactions.
Treatment of positive OFCs was based on the type and severity of the reaction. Treatment options included antihistamines, inhaled salbutamol, systemic steroids, and intramuscular epinephrine (if the patient met the criteria for anaphylaxis).
All positive OFCs were retrospectively classified according to the grading system proposed by B Niggemann and K Beyer.17
This study was approved by the local ethics committee of the institution where it was performed.
Statistical analysis
Statistical analysis was performed using the IBM Statistical Package for the Social Sciences® version 26.0. Pearson’s chi-squared test was used to analyze categorical variables, except when the expected cell count was less than 5, in which case Fisher’s exact test was used. A p-value ≤ 0.05 was considered statistically significant.
Results
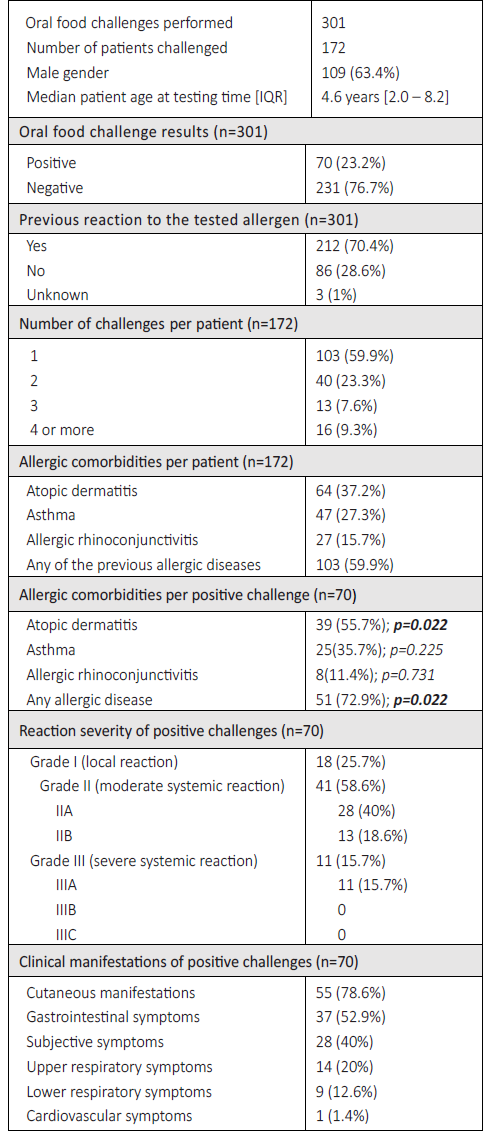
During the 62-month study period, 301 OFCs were performed in 172 patients, 63.4 % of whom were male. At the time of testing, participants ranged in age from six months to 18 years, with a median patient age of 4.6 years (Table 1). Forty percent of patients (n=69/172) underwent two or more OFCs, and a high proportion of patients had other allergic comorbidities (Table 1).
Approximately one quarter of this sample (28.6%; n=86/301) had no history of adverse reactions to the food tested. In these cases, OFCs were mainly performed to assess tolerance status to cross-reactive foods in children with documented food allergies. Of the 86 challenges performed in such cases, 53.5% (n=46/86) were for peanuts and/or tree nuts (in children with known nut allergy) and 11.6% (n=10/86) were for fish (to assess the tolerability of less allergenic fish, such as tuna or skate). The remaining tests were performed in children sensitized to a food for which tolerance was not known (mainly shrimp, in cases of tropomyosin sensitization, and fruits such as kiwi and peach).
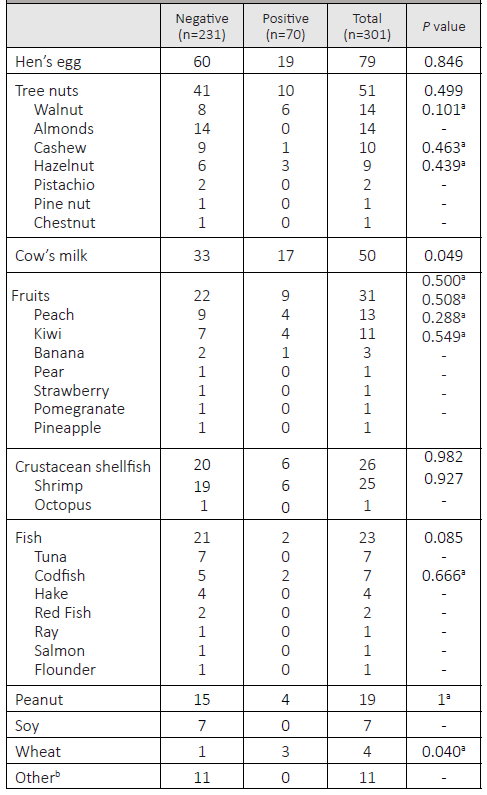
Overall, most tests were negative (n=231/301; 76.7%). Patients who had never reacted to the challenged food had a lower positivity rate (n=14/86; 16.3%) than those who had a history of a previous reaction (n=56/212; 26.4%). These differences were not statistically significant (p=0.061). In terms of allergen type, hen’s egg, tree nuts, and cow’s milk accounted for more than half of all OFCs performed (n=180/301; 59.8%; Table 2).
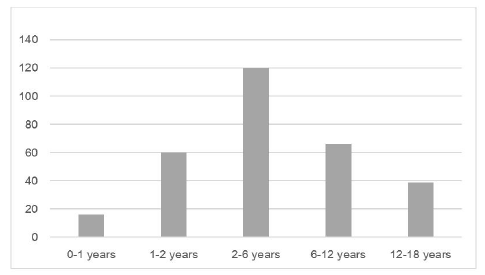
When analyzing positive OFCs by age group, milk and egg were the most common allergens in children up to three years of age, accounting for 18.6% (n=13/70) and 12.9% (n=9/70) of all positive OFCs, respectively. In children aged three to 11 years, eggs remained the most common allergen in positive OFCs (n=10/70; 14.3%), followed by tree nuts and peanuts (n=8/70; 11.4%), and fruits (n=5/70; 7.1%). In adolescents, the main allergens were nuts (n=3/70; 4.3%) and fruits (n=3/70; 4.3%).
Both milk and wheat OFCs were significantly associated with a positive result (p=0.049 and p=0.040, respectively). None of the remaining allergens showed a statistically significant association with the OFC’ results (Table 2).
Regarding the purpose of the OFC, 60.1% (n=181/301) aimed to establish a diagnosis, 25.6% (n=77/301) to assess tolerance, and 14.3% (n=43/301) to document the threshold of responsiveness. Sixty five percent (n=28/43) of the threshold definition tests were positive, followed by 23.4% (n=18/77) of the tolerance tests and only 13.3% (n=24/181) of the diagnostic tests. Considering only the tolerance tests, although not statistically significant, the median patient age in the positive test group was slightly lower than in the negative test group (2.7 vs 3.9 years). Regarding the positive threshold level definition OFC, the median eliciting dose was 1443 mg, and 10 patients consumed the final cumulative dose of 4443 mg before a reaction occurred.
Cutaneous symptoms were the most common manifestation during positive challenges (n=55/70; 78.6%), followed by gastrointestinal symptoms (n=37/70; 52.9%; Table 1). Persistent subjective symptoms (e.g., nausea or itchy throat) were individually responsible for stopping the challenge in two of the 70 positive OFCs.
According to the grading system used, 18 children had local grade I reactions (e.g., erythema, pruritus) and 41 had grade II reactions, either grade IIA (n=28), characterized by either cutaneous or gastrointestinal involvement, or grade IIB (n=13), characterized by simultaneous cutaneous and gastrointestinal involvement (Table 1). Only 11 patients had grade IIIA reactions, mostly due to respiratory symptoms, such as cough (n=8) or stridor (n=2), and in one case due to tachycardia. No grade IIIB or IIIC reactions were observed. Epinephrine was administered 24 times, for all grade IIIA and IIB reactions. No patient required more than one epinephrine administration and no biphasic reactions occured.
Patients with other atopic diseases were significantly more likely to have a positive OFC (p=0.022), especially those with atopic dermatitis (p=0.022). No other associations were found (Table 1).
The severity grading of positive OFCs per allergen is shown in Figure 1. No statistically significant associations were found between the allergen tested and the severity of reactions.
Discussion
This study identified a total of 23.3% positive OFCs, which is consistent with the findings of other authors reporting positivity rates ranging from 9.6% to 33%.18-21 The increased risk of a positive test found in children with atopic dermatitis is also consistent with the reviewed literature.14,21,22) Atopic dermatitis is a known risk factor for the development of food allergy, as the defects in the skin barrier may favor allergic sensitization. In a population-based study, Flohr et al. demonstrated a direct association between early-onset atopic dermatitis and an increased risk of food sensitization at three months of age (up to six times higher than in healthy controls).23 There is also published evidence that the severity of atopic dermatitis correlates with the persistence of food allergy.24,25 Therefore, appropriate treatment of atopic dermatitis may be a preventive strategy for the development of food allergy.26
In contrast to the findings of Abrams and Becker, no significant association was found in this study between the presence of asthma and the likelihood of a positive OFC.21
The most common causes of food allergy in European pediatric patients are hen’s egg and cow’s milk, so it is not surprising that these allergens were among the most frequently identified causes of food allergy in the present study, especially in younger children.3,27) The distribution of the remaining allergens by age group was heterogeneous. In children aged three to 11 years, the main allergens identified were eggs, nuts, and fruits, while a recent Portuguese study reported that fish also figured among the most frequent culprits of food allergy in this age group.9 However, it should be noted that the referred study included a large number of patients who did not undergo OFC. A low rate of OFC positivity was found in adolescents, with nuts figuring as the main allergen, in agreement with a previously mentioned Portuguese study.10
Regarding the purpose of the OFC, as expected, the highest percentage of positive tests was observed in the threshold level definition group, with a positivity rate of 65.1%. Most of these reactions were elicited by the ingestion of large amounts of food protein (median cumulative dose of 1443 mg, with ten patients reaching the maximum dose before experiencing a reaction). Therefore, despite the number of positive challenges, testing still proved to be useful in providing a better understanding of patients’ allergies and contributing to a better quality of life.
Regarding tolerance challenges, about one quarter of these (n=18/77; 23.4%) were positive. These results prompted a revision of the patient selection criteria. Although not statistically significant, the median patient age was found to be lower in the OFC-positive group (2.7 vs 3.1 years). In addition, of the 18 positive tolerance OFCs, 11 were for milk and seven for eggs, both of which are common allergens for which tolerance is acquired mostly throughout infancy.24,28 Therefore, one might infer that early testing, prompted by the nutritional importance of these allergens, may be partially responsible for the percentage of positive results. However, it should be emphasized that most of these patients had mild to moderate reactions (seven grade I, six grade IIA, and five grade IIB).
The high percentage of negative diagnostic OFCs indicates that most of the suspected allergens were being avoided unnecessarily, compromising the patients’ quality of life. Through testing, these children were able to safely include the evicted food in their diets.
A total of 70 OFCs to either tree nuts or peanuts were performed, 64.3% of which in children with a known nut allergy but no history of reaction to the specific agent tested. Nuts are a frequent source of parental anxiety because they are often associated with life-threatening anaphylaxis.29) Avoidance of all nuts is usually recommended in the presence of a single nut allergy, due to the high rate of cross-reactivity between these type allergens and the potential risk of accidental contamination.29,30 This practice prevents patients from knowing their exact diagnostic status, which further contributes to the high psychological burden associated with nut allergy. It may also contribute to risk-taking behavior, as patients may feel that precautionary avoidance of all nuts is unnecessarily restrictive. In the Pediatric Department of this study’s hospital, skin prick testing is combined with molecular allergen analysis to assess which nuts a child may be less likely to react to. Afterwards, OFCs are selectively performed to the nuts most likely to be tolerated. This provides the opportunity for greater diagnostic clarity through a tailored avoidance diet.
In this study, OFCs to both milk and wheat were significantly associated with a positive result. Cow’s milk allergy is one of the most common food allergies in early childhood.3,31 It was the second most tested allergen in this study. When analyzing possible predictive factors for a positive milk test, a higher (although not statistically significant) frequency of atopic conditions was found in the group with a positive result (64.7% vs. 39.4%).
Wheat is also considered one of the eight most important food allergens, and wheat allergy has been implicated in several diseases such as exercise-induced anaphylaxis and Baker’s asthma.32 The prevalence of wheat allergy is estimated to be less than 1%, with most children acquiring tolerance before the age of 10 years.32,33 Although recent studies have sought to define specific cut-off levels for wheat-specific IgE, there are still areas of uncertainty that may correlate with the percentage of positive results obtained.34 It should be noted that the small sample size in this study may hinder the interpretation of this results.
Contrary to what was reported by other authors, no correlation between the severity of positive reactions and food allergens was found in this study.21
There was a low percentage of severe reactions (i.e., grade III) reactions. After a positive result, all patients maintained regular multidisciplinary follow-up with appropriate medical, nutritional, and psychological support.
The authors acknowledge that the present study has limitations. Due to its retrospective nature, the clinical data reviewed relied on the accuracy of medical records. In addition, the data available for analysis (such as allergen-specific IgE levels, type of index reaction, and time from the first reported reaction to testing) could not be consistently obtained for all patients. The size of the study sample was also limited by the temporary closure of all non-urgent procedures during the COVID-19 pandemic. The OFCs performed were open rather than double-blinded and placebo-controlled, which is the gold standard for the diagnosis of food allergy. However, since most of the documented reactions were objective (only two tests were stopped due to purely subjective symptoms), the authors believe that this did not affect the conclusions of the study.
Conclusion
In conclusion, the OFC positivity rate in this study was 23.3%. Children were more likely to experience a reaction if they had concomitant atopic dermatitis. Most reactions were mild to moderate, confirming the safety of the practices conducted. A detailed clinical history is the first step in the assessment of a potential food allergy, followed by skin prick testing and/or the measurement of allergen-specific IgE. Still, OFCs remain the ultimate diagnostic tool to accurately diagnose food allergy.
Authorship
Inês Patrício Rodrigues - Conceptualization of the work, development of the investigation methodology, literature review and writing of the manuscript.
Carlos Rocha-Castro - Formal analysis of the study data and writing of the manuscript.
Miguel Bernardo - Data curation and review and editing of the final manuscript.
Marinela Santos - Review and editing of the final manuscript.
Clara Matos - Review and editing of the final manuscript.
Tânia Monteiro - Oversight for the research activity planning and execution, review and editing of the final manuscript.
Marisa Carvalho - Oversight for the research activity planning and execution, review and editing of the final manuscript.
Márcia Quaresma - Oversight for the research activity planning and execution, development of the investigation methodology, review and editing of the final manuscript.