Introduction
Tuberculosis is a major public health concern. Despite being a curable and preventable disease, it accounted for 1.5 million deaths in 2020, making it the second leading cause of death from a single infectious agent worldwide.1,2) Despite these facts, reliable data on the burden of tuberculosis in adolescents remain scarce, and a significant number of cases are misdiagnosed, particularly in countries with a low incidence of the disease.3,4 Tuberculosis has a wide spectrum of presentations, making diagnosis challenging. It most commonly affects the lungs (82% of cases), but Mycobacterium tuberculosis bacilli can spread hematogenously and cause miliary tuberculosis (less than 2% of cases), a rare but lethal form of tuberculosis if left untreated.5 Neurological involvement has been described in up to 22% of cases of this form of extrapulmonary tuberculosis.6 Miliary tuberculosis usually affects young unvaccinated children and immunocompromised adults, especially those infected with the human immunodeficiency virus.7 The present case has unique features in that it describes a case of miliary tuberculosis in an immunocompetent adolescent vaccinated with the Bacillus Calmette-Guerin (BCG) vaccine.
Case report
A previously healthy 14-year-old girl, born in Brazil and living in Portugal for the past three years, presented to the Emergency Department with a five-month history of generalized fatigue, anorexia, and weight loss that had progressively worsened.
The adolescent also complained of headaches with photophobia, episodes of shortness of breath, and vespertine chills since the previous month, associated with nausea, vomiting, and night sweats since the week before observation. Occasional nonproductive cough was mentioned with one episode of hemoptysis, which was denied by the girl. No recent travel, animal exposure, or contact with known cases of disease were reported. She was vaccinated according to the Brazilian national vaccination schedule, which included BCG vaccination at birth. She had a normal chest radiograph in the first month of symptoms and had been referred to a Child Psychiatry appointment, where she was started on fluoxetine with no improvement. A computed tomography (CT) scan of the head was performed at the beginning of the fifth month of symptoms and was normal. The adolescent had received antiemetic and analgesic medications on three previous visits without improvement.
On admission, she was febrile and emaciated with a total weight loss of 12 Kg compared to her weight one year before. On physical examination, she was dehydrated and pale, with positive meningeal signs and no other relevant changes. Laboratory study revealed microcytic hypochromic anemia (hemoglobin 9.9 g/dL, mean corpuscular volume 74.2 fL, mean corpuscular hemoglobin 31.9 pg, erythrocyte distribution width 15.9%), thrombocytosis (platelet count 617,000/uL), and elevated erythrocyte sedimentation rate (41 mm/h; normal range 0-10 mm/h), C-reactive protein (25 mg/dL; normal range <3 mg/dL), and serum ferritin (352.10 ng/mL; normal range 10-291 ng/mL). Serum electrolytes, glucose, kidney function, liver enzymes, lactate dehydrogenase, total protein, albumin, and coagulation were normal, and serologies for Epstein-Barr virus, cytomegalovirus, toxoplasmosis, Mycoplasma pneumoniae, and Borrelia were negative. Interferon-gamma release assay (IGRA), blood and urine cultures were obtained.
Given the severity of the presentation and the consequent need for prompt and accurate treatment, a thoracoabdominal CT scan was ordered and showed a bilateral micronodular diffuse pattern in the lungs with cavitated nodules in the apex and upper lobes (Figure 1). The diagnosis of miliary tuberculosis was the first hypothesis. A lumbar puncture was performed, which revealed clear cerebrospinal fluid (CSF), pleocytosis of 150 lymphocytes/μL, elevated protein (96.10 mg/dL), and low glucose (38 mg/dL, 35% of the blood level). Chloride was not measured. CSF Polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative. Signs and symptoms of the disease, CSF findings, and thoracic CT findings suggested the diagnosis of miliary tuberculosis, and anti-bacillary drugs (isoniazid, rifampicin, ethambutol, and pyrazinamide) were started.
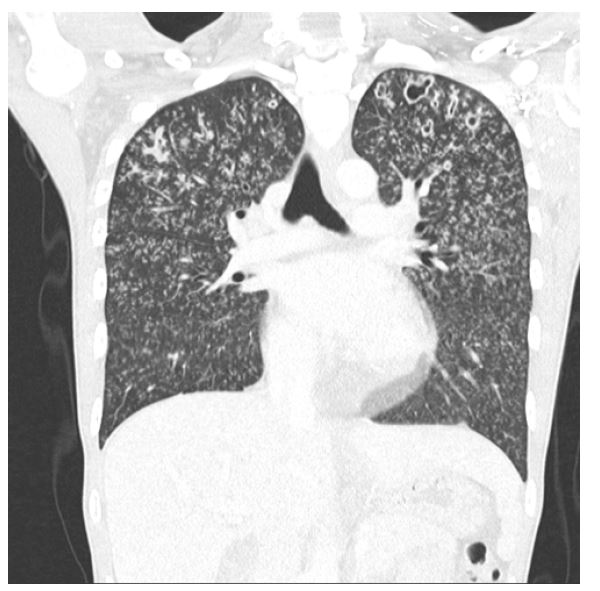
Figure 1 CT scan showing bilateral micronodular diffuse pattern in the lungs with cavitated nodules in the apex and upper lobes.
Sputum smear and bronchofibroscopy for bronchial aspirate and bronchoalveolar lavage (BAL) were performed on the first day of admission. PCR of BAL for Mycobacterium tuberculosis was positive.
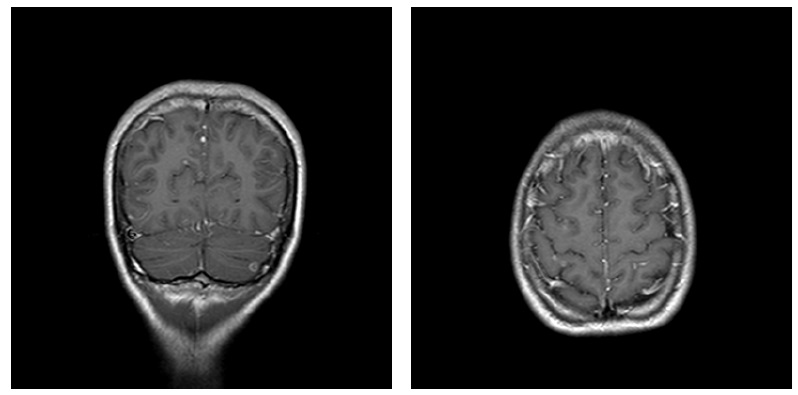
The patient's neurological status worsened within the second day of admission, with intermittent drowsiness. Brain magnetic resonance imaging was performed and showed multiple focal intra-axial lesions most likely compatible with caseous granulomas, focal areas suggestive of vasculitis, and diffuse enhancement of the leptomeninges (Figures 2 and 3). Levofloxacin and intravenous steroids were added to the treatment regimen, and the patient was transferred to a tertiary hospital for continued care.
Levofloxacin was discontinued after the results of anti-bacillary susceptibility testing were known, and steroids were gradually tapered. The patient was discharged approximately one month later with significant clinical improvement. Sputum smear and blood cultures were negative, IGRA was positive, and CSF and BAL mycobacteriology cultures were positive for Mycobacterium tuberculosis. Human immunodeficiency virus infection was ruled out. Immunologic evaluation revealed normal immunoglobulins, lymphocyte immunophenotyping without relevant changes, no overexpression or deficit of CD119 on monocytes, no IL12 receptor deficit in CD25 T lymphocytes, and no interferon gamma deficit at the intracellular level. Next-generation sequencing of genes potentially involved in susceptibility to mycobacterial infection (CARMIL2, CYBB, GATA2, IFNGR1, IFNGR2, IKBKG, IL12B, IL12RB1, IRAK4, IRF8, ISG15, JAK1, NFKBIA, RORC, SPPL2A, STAT1, TYK2) was performed, with no results yet known.
At the follow-up visit 15 days after discharge, the girl had regained her previous weight and was feeling well. She had completed two months of isoniazid, rifampicin, ethambutol, and pyrazinamide without adverse events. Steroids were suspended after two months of therapy with no recurrence of symptoms.
The patient is currently completing ten months of isoniazid and rifampicin, with normal neurologic examination and good school performance with satisfactory grades. Medication compliance is being monitored by direct visualization of administrations by the Pneumological Diagnostic Center of her area of residence. She was referred to Neurology and Ophthalmology and is scheduled for repeat brain MRI. Screening of close contacts (only members of the adolescent's household) was negative.
Discussion
This study reports a case of delayed diagnosis of disseminated tuberculosis in a previously healthy adolescent, highlighting the many barriers clinicians face in diagnosing and managing this disease, particularly in adolescents. In fact, according to the World Health Organization, there is a significant gap in the diagnosis of tuberculosis in children and adolescents, with 2020 estimates indicating only 44% of cases diagnosed.2
There are several reasons for this diagnostic challenge and delay. On the one hand, the incidence of tuberculosis in Portugal has decreased in recent decades, with the disappearance of high-incidence regions.8 However, clinicians must be aware that trends differ between regions and population subgroups. In fact, the notification rate of tuberculosis in the immigrant community is four times higher than the national average, and being an immigrant is one of the main risk factors associated with severe forms of the disease.9) This raises the question of creating screening programs for immigrants, as has been done in several countries with a low incidence of the disease. The patient in the present case was from a country with a high incidence of tuberculosis, where she had lived most of her life. Although she had lived in Portugal for almost three years, it is known that some people can take years to develop active tuberculosis, highlighting the potential benefits of early screening.
Adolescence itself is another factor that can contribute to a delay in diagnosis, as it is a time of many physical, psychological, and social changes. At the onset of symptoms, the adolescent was referred to a Child Psychiatry appointment. Although mental and behavioral disorders are a significant burden in this age group, it is extremely important not to ignore some of the warning signs that were present: generalized fatigue, vespertine chills, anorexia, weight loss, and night sweats. Although often overlooked, adolescents are a high-risk group for disease progression after Mycobacterium tuberculosis bacilli infection.4 In addition, this age group also presents unique features in terms of treatment adherence (which is typically low) and long-term comorbidities of infection, which are largely unknown. Another important feature of this age group, and consistent with the reported case, is that most cases are diagnosed by clinical or radiologic suspicion rather than through contact tracing, which represents a public health challenge.10) In this context, it is important to screen the patient’s contacts for the index case to stop the spread of this potentially fatal disease. In the present case, screening of close contacts was negative, but only household members were tested. Ideally, the adolescent's classmates should also be tested.
The chest radiograph may be normal early in the course of the disease, emphasizing the need to repeat it later or to maintain a high index of suspicion and perform a chest CT. Normal brain CT may also be observed in tuberculous meningitis (30% of cases). Although CT scan may be more easily accessible and a means to exclude hydrocephalus, MRI has a higher diagnostic accuracy. As for PCR, its sensitivity for detecting Mycobacterium tuberculosis in a single CSF sample may be as low as 30%. Sputum culture is more likely to be positive in adolescents, but samples may be negative, as in this case.
This case is an important reminder that progressive complaints associated with weight loss, pulmonary symptoms, and residence in a country with a high incidence of tuberculosis, even up to the previous three years, should raise early clinical suspicion. In this case, the delay of almost six months between symptom onset and diagnosis may explain the disease severity.
In conclusion, it is extremely important to keep this diagnostic hypothesis in mind because of the potentially serious consequences of not initiating treatment promptly. A comprehensive approach that addresses the social determinants of tuberculosis is essential to reduce the burden of the disease, with public health interventions playing an important role.
Authorship
Catarina Pinto Silva - Conceptualization; Writing - original draft
Beatriz de Sousa - Writing - original draft
Joana Lima - Writing - original draft
Sofia Vasconcelos - Writing - review & editing
Alícia Rebelo - Writing - review & editing
Ana Luísa Lobo - Writing - review & editing
Alexandre Fernandes - Writing - review & editing