Introduction
At the end of 2019, a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, causing a cluster of pneumonia cases. This outbreak was later declared a global pandemic by the World Health Organization (WHO) and named coronavirus disease 2019 (COVID-19).1
COVID-19 appears to affect children less severely than adults, with most pediatric cases presenting with only mild symptoms. However, a newly recognized condition called pediatric inflammatory multisystem syndrome (PIMS) in Europe and multisystem inflammatory syndrome in children (MIS-C) in the United States has emerged.
According to the Centers for Disease Control and Prevention (CDC), the diagnosis of MIS-C requires the fulfilment of three criteria:2 patients younger than 21 years presenting with fever (temperature >38ºC for more than 24 hours or subjective fever reported for the same duration), laboratory evidence of inflammation, and severe illness requiring hospitalization with involvement of at least 2 organs; no alternative plausible diagnoses; and evidence of current or recent SARS-CoV-2 infection as demonstrated by reverse transcription polymerase chain reaction (RT-PCR,) serology, or antigen testing or documented exposure to a suspected or confirmed COVID-19 case within 4 weeks prior to symptom onset.
The mechanism of disease in MIS-C is not fully understood, but it is believed to represent a delayed immune response to SARS-CoV-2 infection in children, resulting in exaggerated inflammation and subsequent tissue damage.3
The clinical presentation of MIS-C is broad, but typically includes persistent fever and gastrointestinal symptoms such as vomiting, diarrhea, and abdominal pain. In addition, cardiac and mucocutaneous manifestations are common. (4-6 In severe cases, MIS-C can progress to shock and multiorgan failure, often requiring intensive care.
Initially, MIS-C was thought to be a variant of Kawasaki disease or toxic shock syndrome, and many authors have highlighted the similarities between these conditions.7-9 However, they have significant differences, and MIS-C is now recognized as a distinct clinical entity.
The diagnosis of MIS-C remains challenging, as it is often difficult to differentiate from other invasive febrile diseases. In the Pediatric Intensive Care Unit (PICU) of Hospital de Santa Maria (HSM), a university hospital in Lisbon, a comprehensive laboratory panel is performed on all patients with suspected MIS-C, and some findings are consistent with reports from the broader medical community. However, several abnormal laboratory parameters observed in MIS-C patients are not routinely evaluated in other invasive diseases, leaving it unclear whether these findings are specific to MIS-C.
The aim of this study was to evaluate the laboratory features of MIS-C and explore how it can be distinguished from other diseases with similar presentations. The primary goal is to improve the understanding of this emerging disease in order to optimize its diagnosis.
Patients and methods
Study design
A single-center prospective study was conducted in the PICU of HSM, in Lisbon, Portugal. Two groups were compared: the MIS-C group and the non-MIS-C group. Patients under 18 years who met the inclusion criteria were included in the study. For the MIS-C group, the diagnosis was based on the CDC diagnostic criteria, with all children required to have positive immunoglobulin G (IgG) antibodies for SARS-CoV-2. The non-MIS-C group included children with fever and invasive diseases unrelated to COVID-19 who were admitted to the PICU during a six-month period (October 2021 to March 2022). Inclusion criteria for this group required a diagnosis of invasive febrile disease, excluding postoperative patients.
The same laboratory panel was performed for both groups and included the following parameters: C-reactive protein (CRP), procalcitonin (PCT), erythrocyte sedimentation rate (ESR), interleukin-6 (IL-6), N-terminal pro-B-type natriuretic peptide (NT-proBNP), troponin T, ferritin, activated partial thromboplastin time (aPTT), prothrombin time (PT), fibrinogen, and lymphocyte count. Laboratory analysis was performed twice: on admission and 48 hours later. For all variables except lymphocytes, the highest value recorded during the analysis was selected. For lymphocytes, the lowest value was selected.
This study was approved by the Ethics Committee of the Lisbon Academic Medical Center (reference number 284/21). Informed consent was obtained from the guardians of all participants in the non-MIS-C group.
Statistical analysis
All data were entered into Microsoft Office Excel 2018 and analyzed using IBM SPSS Statistics, version 27.
First, data normality was assessed by comparing the mean and median values and evaluating the skewness and kurtosis (values between -2 and 2 were considered indicative of a normal distribution). For quantitative variables without a normal distribution, the non-parametric Mann-Whitney U test for independent samples was used. Results were summarized using the median and interquartile range (IQR) for comparisons between the two groups. The qualitative variable (gender) was described using counts and percentages. A p-value <0.05 was considered statistically significant.
Results
Patient population
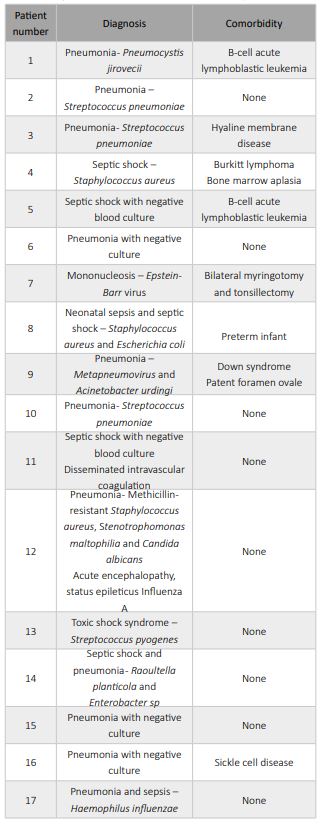
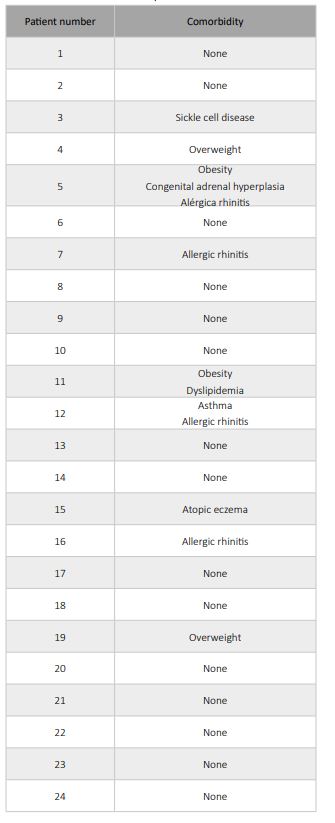
A total of 41 patients were included in the study, 24 in the MIS-C group and 17 in the non-MIS-C group (Table 1). In the non-MIS-C group, the diagnoses included pneumonia (52.9%; n=9), sepsis (41.2%; n=7), and infectious mononucleosis (5.9%; n=1; Table 2). In the pneumonia cases, the causative microorganism was not identified in all cases, but bacterial pneumonia was suspected in all patients. The comorbidities found in the non-MIS-C and MIS-C groups are summarized in Tables 2 and 3, respectively. In the MIS-C group, 62.5% of patients (15 of 24) had no comorbidities (Table 3).
Regarding age distribution, the MIS-C group consisted of older children with a median age of 12 years (IQR: 7-16) compared with to median age of 2 years (IQR: 1-16) in the non-MIS-C group (Table 1). In both groups, most patients were male: 75% (18 of 24) in the MIS-C group and 75% (18 of 24) in the non-MIS-C group (Table 1).
Table 1 Demographic and laboratory characteristics of MIS-C and non-MIS-C patients.
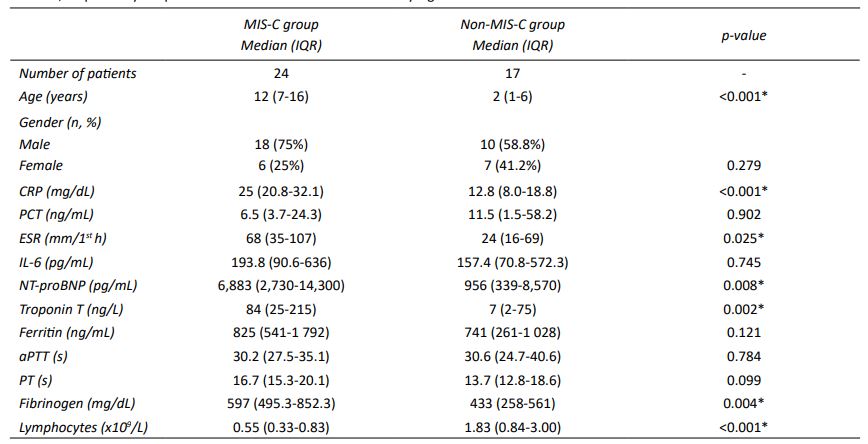
Data are presented as median and interquartile range (IQR = P25-P75). The control values for aPTT and PT were 29 seconds and 11.6 seconds, respectively. *A p-value <0.05 was considered statistically significant.
Laboratory findings
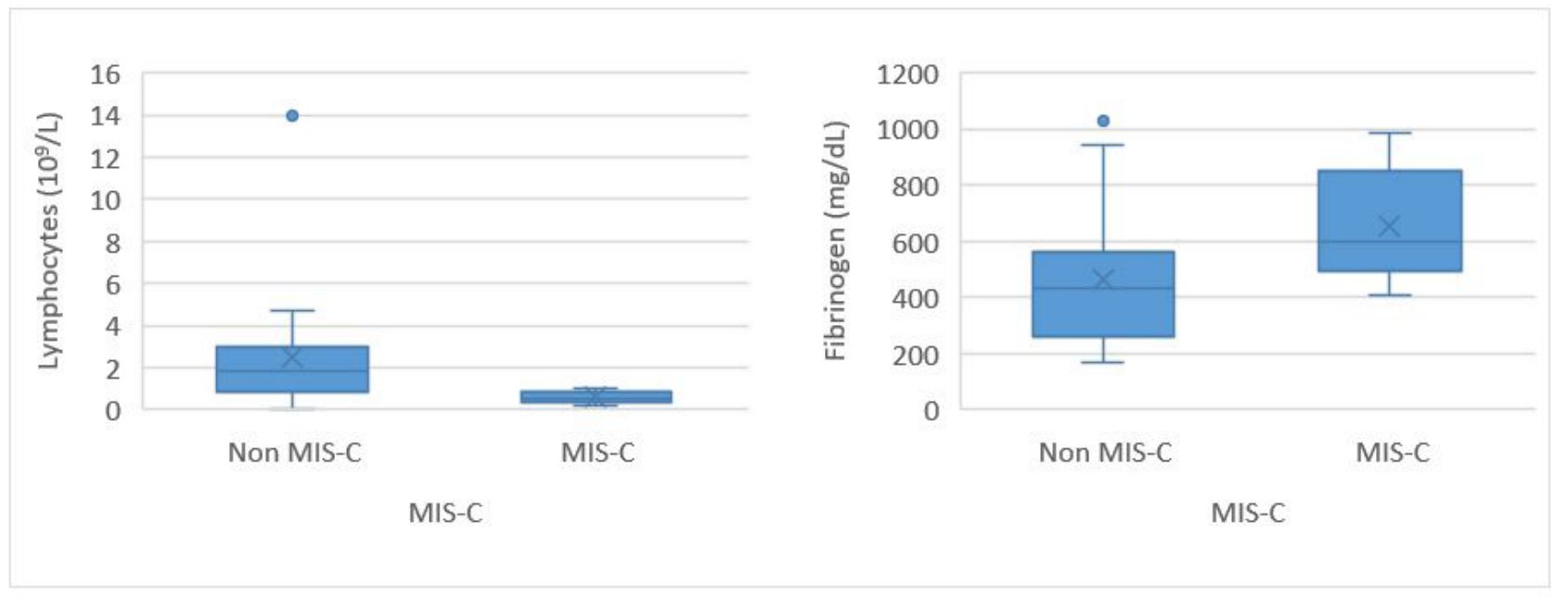
CRP, ESR, IL-6, NT-proBNP, troponin T, fibrinogen, and lymphocytes showed statistically significant differences between the two groups, with all except lymphocytes showing higher levels in the MIS-C group compared with the non-MIS-C group (Table 1). Lymphocyte counts had minimum and maximum values of 0.21 × 10⁹/L and 1.01 × 10⁹/L in the MIS-C group compared to <0.1 × 10⁹/L and 14.0 × 10⁹/L in the non-MIS-C group, respectively. Of note, two patients with bone marrow aplasia had lymphocyte counts <0.1 × 10⁹/L. CRP had a median (IQR) value of 25 (20.8-32.1) mg/dL in MIS-C patients and 12.8 (8.0-18.8) mg/dL in non-MIS-C patients, a difference that was statistically significant (p<0.001; Table 1).
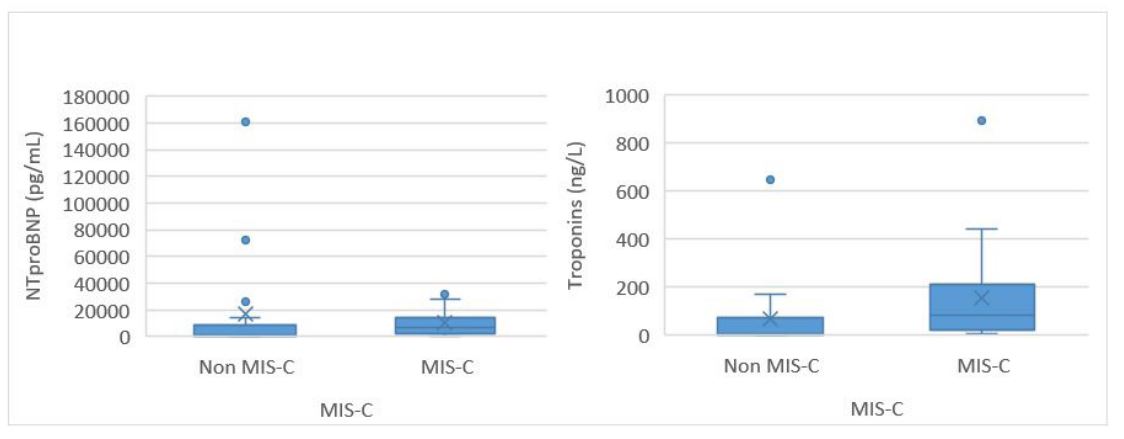
Regarding cardiac markers, median (IQR) NT-proBNP was 6,883 (2,730-14,300) pg/mL (minimum 750 pg/mL; maximum 34,389 pg/mL) in the MIS-C group and 956 (339-8,570) pg/mL (minimum 95 pg/mL; maximum 160,404 pg/mL) in the non-MIS-C group (p=0.008). Troponin T was also higher in the MIS-C group, with a median (IQR) of 84 (25-215) ng/L (minimum 7 ng/L; maximum 892 ng/L) compared to 7 (2-75) ng/L (minimum 1 ng/L; maximum 648 ng/L) in the non-MIS-C group (p=0.002; Table 1).
The remaining laboratory parameters, including PCT, IL-6, ferritin, aPTT, and PT, did not show statistically significant differences between the two groups. However, the values for these parameters were outside the normal range in both groups (Table 1).
Figures 1-3 illustrate the box plots for the parameters that showed statistically significant differences between the two groups.
Discussion
Demographic considerations
The age distribution in the MIS-C group was higher than in the non-MIS-C group, with a median of 12 years. This is consistent with the findings of Williams et al., who reported that MIS-C primarily affects older children, with a median age of 9 years. (6 Although this age difference could potentially influence the results, the laboratory reference ranges were not significantly different between age groups.
In terms of gender distribution, the MIS-C group had a majority of male patients (75%). Although it is not conclusive that this disease affects males more frequently than females, similar trends have been observed in other studies.10),(11
Regarding comorbidities, as shown in Table 3, MIS-C seems to predominantly affect previously healthy children. This observation has been documented in the literature and contrasts with COVID-19, which tends to be more severe in children with underlying comorbidities.
C-reactive protein and erythrocyte sedimentation rate
CRP is an acute-phase protein produced by the liver that rises significantly in response to inflammation and infection.12 Elevated ESR is associated with conditions characterized by elevated protein or fibrinogen levels, such as autoimmune or cardiovascular diseases.13
As shown in Figure 1, the results of this study indicate that CRP and ESR levels are significantly higher in the MIS-C group compared to the non-MIS-C group. This finding suggests that MIS-C is associated with a higher degree of inflammation than other invasive diseases. This increased inflammatory response is similar to that observed in Kawasaki disease and toxic shock syndrome, two conditions often compared to MIS-C. In fact, MIS-C was originally described as a variant of these diseases associated with SARS-CoV-2.14) Toxic shock syndrome is triggered by bacterial superantigens produced by the causative organism. These superantigens mediate T-cell activation, resulting in a cytokine storm.15 In contrast, Kawasaki disease is an immune-mediated vasculitis characterized by a pro-inflammatory state. While the role of superantigens in Kawasaki disease remains controversial, it is now believed that T-cell activation occurs via a conventional antigen-mediated mechanism.8),(16),(17
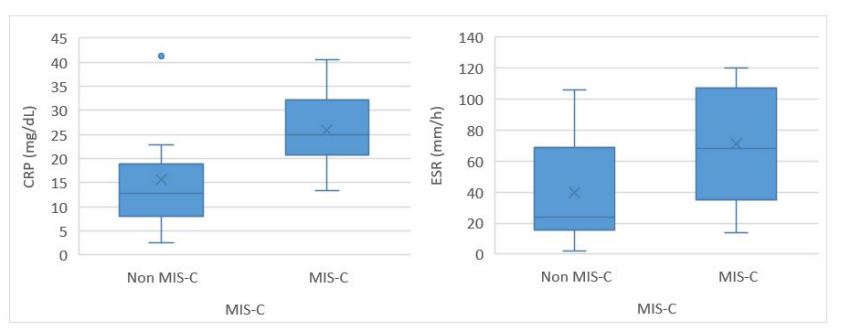
Figure 1 Box plots of C-reactive protein and erythrocyte sedimentation rate levels in non-MIS-C and MIS-C patients. RP, C-reactive protein; dL, deciliter; ESR, erythrocyte sedimentation rate; h, hour; MIS-C, multisystem inflammatory syndrome in children; mm, milimiter.
Regarding the mechanism of disease in MIS-C, Porritt et al. described the expansion and activation of a specific class of T cells as a key pathophysiological mechanism driving the development of a cytokine storm.18 In addition, they identified an HLA association in MIS-C cases that may explain why only a small proportion of children infected with SARS-CoV-2 develop this severe inflammatory syndrome. Another study suggested that the SARS-CoV-2 spike protein may act as a superantigen, triggering the hyperinflammatory response observed in MIS-C and accounting for its similarity to toxic shock syndrome.19
NT-proBNP and troponin T
Cardiac involvement in MIS-C has been extensively documented, with some patients presenting primarily in cardiogenic shock. Monitoring of cardiac enzymes in patients with suspected or confirmed MIS-C is critical, as cardiac involvement plays a significant role in disease progression and management, and in prognosis prediction.20 Mannarino et al. reported that ventricular dysfunction, pericardial effusion, and valvulitis are commonly diagnosed in patients with MIS-C.21 They concluded that although the heart is frequently affected in MIS-C, cardiac involvement does not adversely affect patient prognosis if properly treated and supported during the acute phase.
The results of this study indicate that cardiac markers were significantly higher in the MIS-C group (Figure 2). In particular, the minimum NT-proBNP level for this group was 750 pg/mL, indicating that all MIS-C patients in the study had some degree of cardiac involvement. This finding is supported by numerous studies that have reported echocardiographic abnormalities in MIS-C patients.6),(10
Fibrinogen
Fibrinogen is an important indicator of coagulopathy. In invasive diseases such as sepsis, a systemic inflammatory response and vascular endothelial cell injury can result in abnormal coagulation values. Tang et al. demonstrated that fibrinogen may be a valuable prognostic biomarker in pediatric sepsis, as lower fibrinogen levels were associated with a higher mortality rate.22
In MIS-C, fibrinogen levels are typically elevated, a finding supported by the results of this study, as fibrinogen levels were significantly higher in the MIS-C group (Figure 3). (23 This may be due to the role of fibrinogen as an acute phase reactant during systemic inflammation. In contrast, the other coagulation parameters, aPTT and PT, did not show significant differences between the two groups.
In critical situations such as sepsis or shock, fibrinogen is consumed, especially in the presence of disseminated intravascular coagulation. This is not the case in MIS-C, where fibrinogen levels are elevated. The difference in fibrinogen levels between MIS-C and other invasive diseases could be used in the differential diagnosis, suggesting that if a patient with suspected MIS-C presents with low fibrinogen levels, the diagnosis of MIS-C is less likely.
Lymphocytes
Lymphopenia was highly significant in the MIS-C group, with lymphocyte counts being relatively consistent across patients. Notably, there was minimal variation among the 24 values, as shown in Figure 3.
In a comparative study by Yakut et al., lymphopenia was found to be significantly more common in the MIS-C group compared to children diagnosed with COVID-19,10 with a reported median lymphocyte count of 1.8 × 10⁹/L.9
In this study, all MIS-C patients showed significant lymphopenia, further highlighting the relevance of this parameter as a potential diagnostic marker in MIS-C. This finding could be a valuable tool in differentiating MIS-C from other invasive diseases. If a patient with suspected MIS-C presents without lymphopenia, the diagnosis becomes highly unlikely and an alternative diagnosis should be considered.
However, lymphopenia is also frequently observed in severe sepsis, as demonstrated in this study. Although the median lymphocyte count in the non-MIS-C group was higher than in the MIS-C group, it was still markedly low.24 Therefore, the differential diagnosis with severe sepsis should still be carefully considered in cases where a patient with suspected MIS-C presents with lymphopenia.
Interleukin-6
IL-6 is a pro- and anti-inflammatory cytokine with very low serum levels under normal conditions. However, these levels usually increase in inflammatory states, especially in autoimmune diseases.25
Some authors have described elevated serum levels of IL-6 in MIS-C. For example, DeBiasi et al. performed a prospective observational cohort study comparing a group of MIS-C children with a group with similar but different diagnosis and found that the MIS-C group had a higher plasma IL-6 concentration than the non-MIS-C group.26 Diaz et al. conducted a retrospective cohort study comparing plasma IL-6 concentration between MIS-C and pediatric sepsis patients.27 The authors showed that although plasma IL-6 concentration was elevated in both groups, the value was higher in the septic shock population compared with the MIS-C population, contrary to what the authors had predicted.
In the present study, although IL-6 levels were elevated in both groups, no significant difference was found between them. However, this elevation of plasma IL-6 concentration remains relevant, as it supports the previously discussed cytokine storm hypothesis.
IL-6 is rarely included in routine laboratory panels for patients with invasive febrile diseases. The results of this study show that IL-6 levels are elevated in a variety of invasive disease states, suggesting that it is not specific to MIS-C.
Tocilizumab, an IL-6 receptor antagonist, has been used in the treatment protocol for severe COVID-19 cases, with a cut-off of 40 pg/mL used to initiate treatment in HSM. (28 However, this study suggests that this cut-off may be too low, as median IL-6 levels were found to be significantly higher in both groups.
Procalcitonin and ferritin
PCT and ferritin were not significantly different between the two groups studied and presented median values that were not within the normal range of these parameters, as expected in invasive diseases such as sepsis or even shock.29
PCT is produced in the thyroid gland and is typically undetectable in the serum of healthy individuals under normal conditions. It is a valuable tool in the differential diagnosis of severe bacterial infections and other potential causes.30 This study found that although PCT levels were elevated in both groups, there was no significant difference between them.
It is interesting that although almost all non-MIS-C patients had bacterial infections and MIS-C patients did not, PCT levels were not higher in the non-MIS-C group. This emphasizes that PCT, although useful in the differential diagnosis, is not specific for bacterial infections, as it can also be elevated in cases of multisystemic inflammation such as MIS-C.
Ferritin is an acute phase reactant and is widely used as a serum biomarker.31 Although it is not specific to any single disease, ferritin levels are typically elevated in inflammatory or infectious conditions. In this study, the median ferritin levels were similar between the two groups, with no statistically significant difference. These findings suggest that ferritin is elevated in MIS-C in a non-specific manner, similar to other severe diseases.
Final considerations
MIS-C primarily affects older children and adolescents and is characterized by a hyperinflammatory state with elevated levels of CRP and ESR and a cytokine storm similar to that seen in Kawasaki disease and toxic shock syndrome. Although IL-6 is elevated in MIS-C, it is not specific to this syndrome, as it is also elevated in other severe invasive diseases.
Awareness of cardiac involvement in MIS-C is critical. All patients with suspected or confirmed MIS-C should be closely monitored for cardiac complications and treated promptly to reduce risks and improve outcomes.
A very interesting finding of this study was that lymphopenia is highly characteristic of MIS-C and may be a useful diagnostic tool for early detection of MIS-C and eventually to exclude MIS-C in patients presenting without lymphopenia.
Although PCT is elevated in MIS-C, it rises to levels similar to those observed in bacterial infections. Therefore, in patients with suspected MIS-C, high serum PCT levels do not exclude the diagnosis of MIS-C.
This study aimed to analyze the laboratory profile of MIS-C, but an interesting observation emerged from the non-MIS-C group, which showed unexpected findings such as elevated IL-6 levels. These results provided valuable insights into the laboratory characteristics of other invasive febrile diseases, thus improving the understanding of their inflammatory profiles.
This study has some limitations that should be acknowledged. The most important were the small sample size and the single-center design, which limited the ability to perform subgroup analyses. There were also important limitations regarding the control group. First, the fact that two patients in this group had bone marrow aplasia, which could affect the comparison of lymphopenia between groups. Second, the fact that the control group included one patient with a fungal infection (Pneumocystis pneumonia) and another with a viral infection (infectious mononucleosis), which may have resulted in lower inflammatory markers compared to bacterial infections. Finally, the fact that the control group included a neonate, which may also have affected the evaluation of inflammatory markers due to age-related physiological differences in the immune response.
In conclusion, understanding the laboratory characteristics of MIS-C and other severe invasive conditions such as sepsis is crucial for accurate differential diagnosis. Future studies should focus on investigating the impact of specific laboratory parameters on patient prognosis, identifying biomarkers of severity, and identifying the most effective therapeutic strategies.
Conclusions
Multisystem inflammatory syndrome in children (MIS-C) is a newly recognized and severe condition that remains incompletely understood. The hyperinflammatory response observed in MIS-C can be life-threatening and requires prompt identification and treatment.
Clinicians must remain vigilant for this syndrome in previously healthy school-aged children who present with high inflammatory markers, elevated cardiac enzymes, and lymphopenia. However, laboratory results should be interpreted with caution as these features may overlap with other invasive febrile diseases. In particular, IL-6 levels should be carefully evaluated when making therapeutic decisions, as they do not differ significantly between MIS-C and other febrile diseases.
Finally, raising awareness of this emerging entity is essential. Further research is needed to better understand the pathophysiology of MIS-C, to develop effective therapeutic strategies, and to identify reliable prognostic biomarkers.
Authorship
Inês Tovim - Formal Analysis; Investigation; Methodology; Data Curation; Visualization; Writing - original draft
Francisco Abecasis - Conceptualization; Methodology; Investigation; Formal analysis; Resources; Supervision; Visualization; Validation; Writing - review & editing
Abbreviations
aPTT - Activated partial thromboplastin time
CDC - Centers for Disease Control and Prevention
COVID-19 - Coronavirus disease 2019
CRP - C-reactive protein
ESR - Erythrocyte sedimentation rate
HSM - Hospital de Santa Maria
IL-6 - Interleukin-6
NT-proBNP - N-terminal pro-B-type natriuretic peptide
MIS-C - Multisystemic inflammatory syndrome in children
MRSA - Methicillin-resistant Staphylococcus aureus
PICU - Pediatric Intensive Care Unit
PIMS - Pediatric inflammatory multisystem syndrome
SARS-CoV-2 - Severe acute respiratory syndrome coronavirus 2
SPSS - Statistical Package for the Social Sciences
PCT - Procalcitonin
PT - Prothrombin time
TSS - Toxic shock syndrome
WHO - World Health Organization