Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.32 no.1 Coimbra jan. 2014
https://doi.org/10.4152/pea.201401001
Phenolic and non-Phenolic Fractions of the Olive Oil Mill Wastewaters as Corrosion Inhibitor for Steel in HCl medium
D. Bouknanaa,b , B. Hammoutia,c,* , M. Messalid , A. Aounitia and M. Sbaab
a LCAE-URAC18, Faculté des Sciences d'Oujda, Université Mohammed Premier, BP 4808, 60046 Oujda, Morocco
b COSTE, Faculté des Sciences d'Oujda, Université Mohammed Premier, BP 4808, 60046 Oujda, Morocco
c Department of Chemistry, College of Science, King Saud University, B.O. 2455, Riaydh 11451, Saudi Arabia
d Chemistry Department, Faculty of Science, Taibah University, Al Madinah Al Mounawara, 30002 Saudi Arabia
Abstract
The effect of the phenolic (OOMW-Ph) and non-phenolic (OOMW-NPh) fractions of the extract of olive oil mill wastewaters was evaluated as corrosion inhibitor of steel in molar hydrochloric using weight loss measurements and electrochemical polarisation. The results obtained reveal that the referred compounds reduce the corrosion rate. The inhibiting action increases with the concentration of the extract compounds to attain 88.9% and 89.1% of OOMW-Ph and OOMW-NPh, respectively. The increase in temperature leads to a decrease in the inhibition efficiency of the compounds in the temperature range 303 at 333 K. The adsorption isotherm of the inhibitors on the steel surface has been determined. The thermodynamic data of activation and adsorption are determined as well.
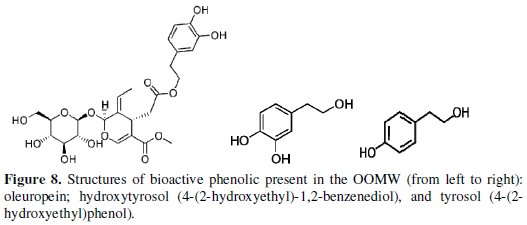
The phenolic compound (bioactive) most abundant in OOMW extracts is hydroxytyrosol (4 -(2-hydroxyethyl) -1, 2-benzenediol), playing an important role in the effect of the anti-corrosion, either alone or in synergy with other two compounds (tyrosol and oleuropein (4 -(2-hydroxyethyl) phenol) which are present with considerable amounts.
Keywords: phenolic fraction, non-phenolic fraction, extract of olive oil mill wastewaters, inhibition, corrosion, steel, acid, hydroxytyrosol, oleuropein, tyrosol.
Introduction
The industrial sector is strongly affected by corrosion which can cause waste of raw materials and energy, creates accidents with serious consequences, and contributes to the pollution of the natural environment [1]. Among various methods of protection, the use of inhibitors which play an important role in the fight against corrosion, protecting and mitigating strategies to reduce the rate of dissolution of metals [2], that is to say, to reduce the rate of either anodic oxidation or cathodic reduction or both, the organic compounds containing hetero atoms (N, O, P or S) are effective corrosion inhibitors for corrosion of various some synthetic corrosion inhibitors have been identified to be toxic and non-ecofriendly [11-12], due to the several negative effects they have caused in the environment [13]. The toxic effect does not only affect living organisms, but also poisons the environment [14]. Thus, search for eco-friendly and non-toxic corrosion inhibitors of natural sources has been considered to be more important and desirable [15]. On account of its availability in the main producing countries of olive oil, and its effectiveness in the struggle against corrosion, olive oil mill wastewater may replace synthetic inhibitors that are known by their toxic effects [16]. The results of the antIcorrosion effect obtained from olive oil mill wastewater [16], encouraged us to test the extracts of phenolic and non-phenolic fractions. To the best of our knowledge, there is no report of the effect of the addition of the extract of the olive oil mill wastewater on the corrosion of C38 steel alloy in HCl solution.
Important quantities of phenolic wastes are produced from several industrial processes in Mediterranean countries each year during the season of production of olive oil. Olive mill wastewater (OMW) is a natural source with high amounts of bioactive substances with attractive properties [17], such as polyphenolic mixtures (4-10 g.L-1) with different molecular weights [18].
The olive fruit is very rich in phenolic compounds. Enzymatic and/or chemical reactions occurring along the olive oil extraction process result in several modifications of the phenolic compounds. The hydrolysis of the glycosidic bonds and the opening of secoiridoids ring are commonly reported [19-20]. The phenolic fraction of OMW is extremely complex, as demonstrated by several authors such as [17, 21-24]; many compounds are still unidentified, and more than twenty biphenyl have been identified recently via LC-MS-MS techniques, including, cinnamic acid derivatives (such as caffeic, coumaric and ferulic acid), benzoic acid derivatives (such as protocatechuic, hydrobenzoic, vanillic and gallic acid) and β-3,4-dihydroxyphenyl ethanol derivatives (such as p-tyrosol and hydroxytyrosol [22, 25-29]. After the olive oil extraction process, only 1 to 2% of the total phenolic contents of the olive fruit passes in the oil phase, while the most part of the olive phenols, about 98% , in fact remain in the wastewater and also in solid wastes. In olive mill waste (OMW) (approximately 53%) and in the pomace (approximately 45%) [30-34], hydroxytyrosol, tyrosol, oleuropein and caffeic acid are the major phenolic components [17].
The objective of this research is to determine and compare the anti-corrosive effect for both phenolic and non-phenolic fractions of olive oil mill wastewater (OOMW) as inhibitor of corrosion of steel in an acid medium (HCl 1 M). The electrochemical behavior of C38 steel in HCl medium in the absence and presence of OOMW was studied by gravimetric and electrochemical techniques such as potentiodynamic polarization, linear polarization and impedance spectroscopy (EIS). The effect of temperature is also studied; it was also the aim of this study to test the experimental data with several adsorption isotherms at different temperatures to determine the standard free energy (Ea) of the adsorption process and get more information about the mode of inhibitor adsorption on the electrode surface (adsorption isotherm).
Experimental details
Samples and materials
The aggressive solution (1 M HCl) was prepared by dilution of analytical grade 37% HCl with bidistilled water; steel samples containing 0.09 % P, 0.38 % Si, 0.01 % Al, 0.05 % Mn, 0.21 % C, 0.05 % S and the remainder iron were used; prior to all measurements, the steel samples have been polished with different emery papers up to 1200 grade, washed thoroughly with bidistilled water, degreased, and dried with ethanol acetone.
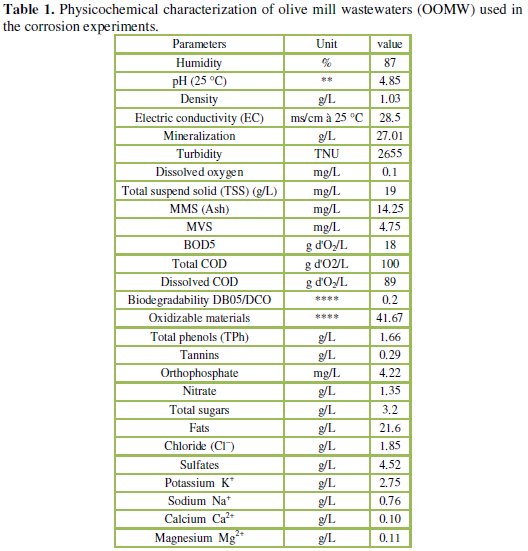
The olive oil mill wastewaters (OOMW) samples used in this study were taken from a three phase olive mill located in the region of Taourirt, eastern of Morocco. The sample of OOMW has been obtained from olives well ripened, collected in November 2013. The effluent was subject to several consecutive filtrations to remove most of the suspended solids (TSS) and its main properties are given in Table 1.
Gravimetric measurements are carried out in a double walled glass cell equipped with a thermostatic cooling condenser. The solution volume is 100 cm3. The steel specimens used have a rectangular form (2 cm × 1.8 cm × 0.3 cm). Electrochemical trends are carried out in a conventional three electrode cylindrical glass cell. The working electrode, in the form of a disc cut from steel, has a geometric area of 1 cm2. A saturated calomel electrode (SCE) and a platinum electrode are used as reference and auxiliary electrodes, respectively. The temperature is thermostatically controlled at 308 K. The polarisation curves are recorded with a potentiostat type EG and G 273, at a scan rate of 30mV/min. The steel electrode was maintained at corrosion potential for 30 min and thereafter pre-polarised at - 800 mV for 10 min. The potential was swept to anodic potentials. The test solution is de-aerated for 30 min in the cell with pure nitrogen which is maintained throughout the experiments
Extraction of phenolic compounds from OMWW
Several protocols have been developed for extract the phenolic compounds from olive oil mill wastewaters (OOMW). They are different from each other by the complexity of the protocol used and efficiency; liquid-liquid extraction was chosen for its simplicity and convenience [17]. The liquid-liquid extraction of the samples of olive mill waste water from its transformation with continuous olive ethyl acetate was carried out according to the protocol described by [35], but modified to meet our objective in the study of corrosion; 50 mL of (OMWW) centrifuged for 30 min at 4600 rpm in order to remove the total suspend solid (TSS) have been used; the supernatant was acidified to pH 2 with HCl and washed with hexane in order to remove the lipid fraction; the mixture was vigorously shaken and centrifuged for 30 min at 4600 rpm; the phases were separated and the washing was repeated successively three times; extraction of phenolic compounds was then carried out with ethyl acetate: 20 mL of the OMWW samples preventively washed were mixed with 10 mL of ethyl acetate; the mixture was vigorously shaken and centrifuged for 30 min at 4600 rpm; the phases were separated and the extraction was repeated successively three times. The ethyl acetate was evaporated under vacuum, the dry residue was dissolved in 10 mL of water bidistilled and this solution was used for study of the corrosion in 1 M HCl medium.
Extraction yield
The extraction yield is calculated by the formula given by Falleh et al. [36], Eq. (1):
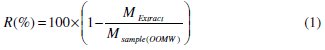
where R is the yield %, Mextract is the mass of the extract after evaporation of the solvent in mg, and Msample is the dry mass of the plant sample in mg; Niaounakis & Halvadakis reported the phenolic extraction yield was equal to 1- 2% of wastewater [34]: we reached the extraction yield of 1.7%.
Dosage of the polyphenols
The total phenol content of olive oil mill wastewaters was determined calorimetrically using the Folin-Ciocalteu reagent [37]. An aliquot of the sample of the olive oil mill wastewaters standard was diluted, then mixed with the Folin- Ciocalteu reagent and 1 mL of a sodium carbonate saturated solution. The final solution was left in the dark for 1 h, after which the absorbance of the solution was measured at 725 nm and compared against a blank prepared following the same protocol but without any sample. A range of gallic acid concentrations from 0.0005 to 0.02 g/mL was used to prepare the calibration curves; total phenol values were expressed as gallic acid equivalents mg/L.
Results and discussion
Weight loss measurements
Table 2 resumes the corrosion rate obtained in 1 M HCl (W0corr) and at various contents of the phenolic fraction (OOMW-Ph) and the non-phenolic fraction (OOMW-NPh) of the extract of olive oil mill wastewaters (Wcorr) determined at 308 K after 6h of immersion rate; the inhibition efficiencies Ew were determined by the relation (Eq. (2)):
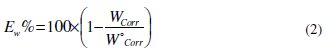
Wcorr and W0corr are the corrosion rates of steel with and without (OOMW-Ph) and (OOMW-NPh) fractions of olive oil mill wastewaters, respectively.
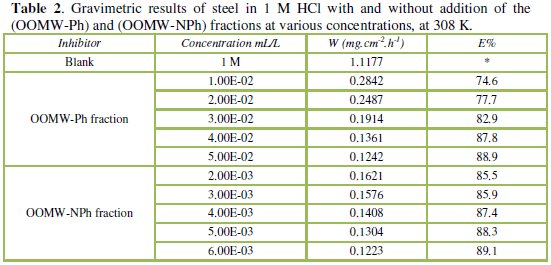
The additions of both phenolic and non-phenolic fractions reduce the corrosion rate in HCl solution. The inhibitory effect increases with the increase of fractions concentration; E% reaches a maximum of 88.9 % at 5.10-2 mL/L and 89.1% at 6.10-3 mL/L for (OOMW-Ph) fraction and (OOMW-NPh) fraction, respectively. The effectiveness of the (OOMW-Ph) and (OOMW-NPh) fractions is due to the presence of heteroatoms such as N, O, S. Because, on the one hand, the physicochemical composition of olive oil mill wastewater is rich in organic matter, and secondly, even if the phenolic compound, that is rich in heteroatoms, is isolated, the non-phenolic fraction is still rich in these compounds as protein fraction. However, we can classify two fractions tested according to the efficiency of growth inhibition as follows: (OOMW-Ph)< (OOMW-NPh).
Finally, we can conclude that both phenolic and non-phenolic fractions of olive oil mill wastewaters extract are good inhibitors of corrosion of steel in 1 M HCl. The non-phenolic fraction achieves high efficiencies even at low concentrations. Therefore, this fraction also contains components playing the role corrosion. If we compare the effectiveness of two fractions for the same concentrations, we can say, in general, that the phenolic fraction participates with 15 to 20 % in the corrosive effect of olives oil mill wastewaters.
Electrochemical polarisation measurements
The cathodic and anodic polarization curves of C38 steel in 1 M HCl in the absence and presence of both phenolic (OOMW-Ph) and non-phenolic fractions (OOMW-NPh) of olive oil mill wastewaters (OOMW) at different concentrations, at 308 K, are presented in Fig. 1.
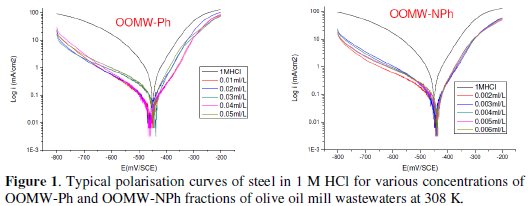
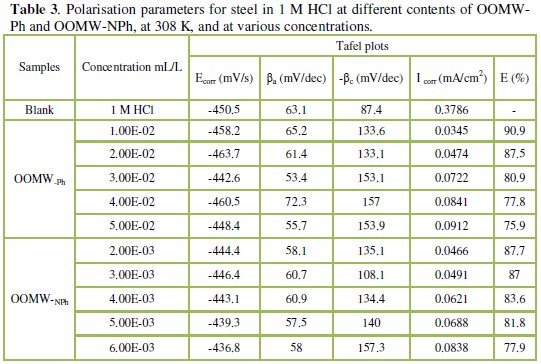
Table 3 gives the values of corrosion current (Icorr), corrosion potential (Ecorr), and cathodic Tafel slope (βc) for both phenolic and non-phenolic fractions of olive oil mill wastewaters in 1 M HCl.
In the case of polarisation method, the relation (3) determines the inhibition efficiency (E %):
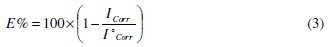
I0corr and Icorr are the uninhibited and inhibited corrosion current densities, respectively, determined by extrapolation of the cathodic Tafel lines to corrosion potential.
The recording of the anodic and cathodic polarization curves has been conducted to obtain information about the action of the inhibitor on the partial corrosion processes; the examination of Fig.1 and Table 3 shows that the addition of OOMW-Ph and OOMW-NPh fractions decreases the current density. The decrease is more pronounced with the increase of the inhibitor concentration; Tafel plots indicate that the mechanism of hydrogen reduction is activation control; the presence of OOMW does not affect the anodic Tafel slope, indicating that the mechanism of H+ oxidation is not modified with the OOMW-Ph and OOMW-NPh concentration.
The polarization curves of steel in molar HCl with and without the phenolic and non-phenolic fractions of the olive mill wastewaters show that the addition of the inhibitor decreases low the anodic current densities in the studied domain of potential for the phenolic fraction (OOMW-Ph), but not for non-phenolic fraction (OOMW-NPh). It could be concluded that the presence of OOMW don't affect the anodic dissolution of steel. The mode of action for phenolic fraction is slightly but significantly anode-cathode, that is to say, slightly mixed, but for the non-phenolic fraction the mode of action of the inhibitor is perfectly cathodic. The inhibition efficiency reaches 90.9 % at 10-2 mL/L concentration and 87.7 % at 2.10-3mL/L of OOMW-Ph and OOMW-NPh, respectively, This phenomenon is interpreted by the adsorption of both fractions on steel surface leading to the increase of the surface coverage, θ, defined by E% / 100. E% increases with the compound concentration. We may conclude that OOMW-Ph and OOMW-NPh are effective inhibitors of steel corrosion in molar HCl.
Electrochemical impedance spectroscopy measurements (EIS)
The corrosion behavior of steel in acidic solution 1 M HCl in the absence and presence of the phenolic fraction (OOMW-Ph) and non-phenolic fraction (OOMW-NPh) of olive mill wastewater was also investigated by (EIS) method, at 308 K, after 30 min of immersion.
The inhibition efficiency can be calculated by the following formula, eq. (4):

where Rt and R0t are the charge transfer resistances in inhibited and uninhibited solutions, respectively.
The values of the polarization resistance were calculated by subtracting the high frequency intersection from the low frequency intersection [38]; double layer capacitance values were obtained at maximum frequency (fm), at which the imaginary component of the Nyquist plot is a maximum, and calculated using eq. (5):

with Cdl double layer capacitance (μF.cm-2), fm maximum frequency (Hz), and Rt charge transfer resistance (Ω.cm 2).
The impedance parameters derived from these investigations are listed in the Table 4.
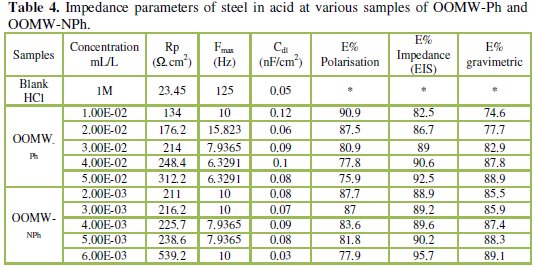
It is visible from Fig. 2 that the obtained impedance diagrams for both phenolic and non-phenolic fractions of olive oil mill wastewaters have a semi-circular appearance, indicating that a charge transfer process mainly controls the corrosion of steel [39].
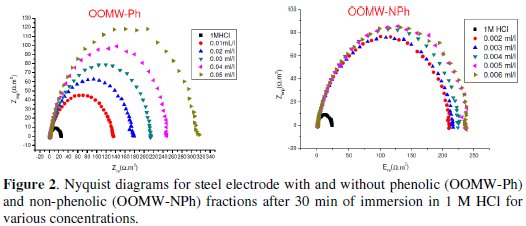
In fact, the presence of phenolic and non-phenolic fractions enhances the value of the transfer resistance in acidic solution; EIS study shows that both fractions tested are efficient inhibitors. The general shape of the curves is almost similar for both fractions; the shape is maintained throughout the whole concentration, indicating that almost no change in the corrosion mechanism occurred due to the inhibitor addition [40]; Rt values increased with the increase of the concentration of the OOMW-Ph and OOMW- NPh fractions of olive oil mill wastewaters. The results obtained from the polarization technique in acidic solution were in good agreement with those obtained from the electrochemical impedance spectroscopy (EIS). Also, inhibition efficiency values obtained from the gravimetric method agree with those obtained from the Tafel extrapolation (Table 4).
Effect of temperature
Weight loss, corrosion rates and inhibition efficiency
The composition of the medium and its temperature are essential parameters affecting the phenomenon of corrosion. The effect of the absence and presence of both phenolic and non-phenolic fractions (OOMW-Ph and OOMW-NPh) of the olive oil mill wastewaters tested at various concentrations during 1 h of immersion on the corrosion of steel in 1 M HCl solution was studied by using weight-loss method from 303 K to 333 K. The inhibition efficiency Ew(%) is calculated at various concentrations as follows (Eq. (6)).
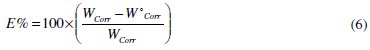
where both Wcorr and W0corr are the corrosion rate of steel in 1 M HCl in the absence and presence of OOMW-Ph and OOMW-NPh inhibitors, respectively.
The corresponding data are shown in Tables 5 and 6.
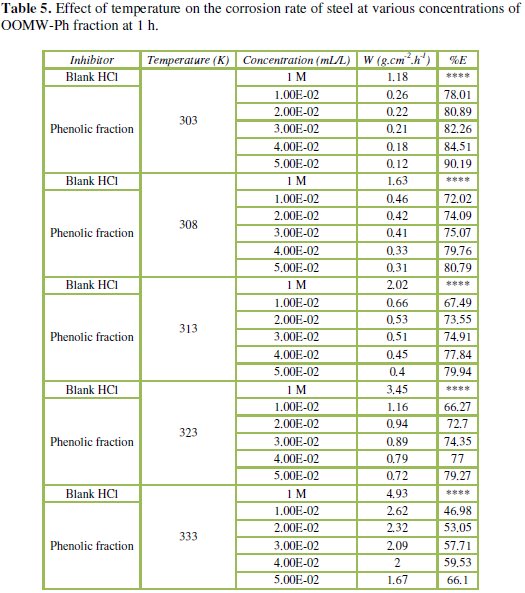
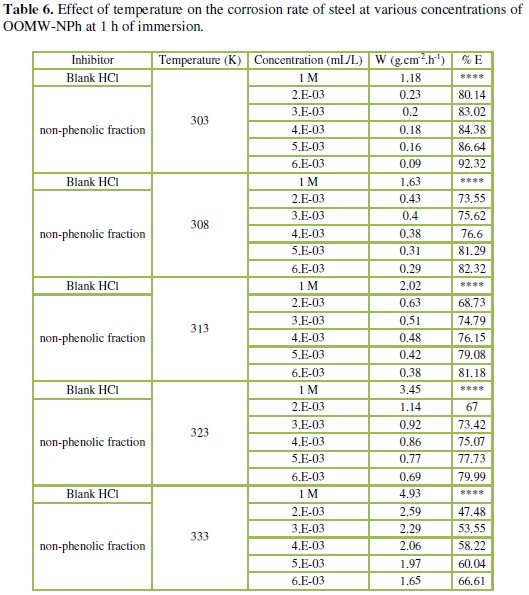
Results in Tables 5 and 6 show that the corrosion rates of steel in 1 M HCl are lower in the presence of both phenolic and non-phenolic fractions of the olive oil mill wastewaters compared to the blank acid solution; also, the corrosion rate increases with increase in temperature with the highest values obtained at 333 K; the inhibition efficiency decreases with increasing temperature, indicating that at higher temperatures, dissolution of steel predominates on inhibitor adsorption. E% is still significant even at high temperature (66.1% for phenolic fraction at a concentration of 5.00E-02 mL/L, and 66.6% for the non-phenolic fraction a concentration of 6.00E-03 mL/L at 333 K); this suggests possible desorption of some of the adsorbed inhibitors from the metal surface at higher temperatures. Such behavior shows that the additive was physically adsorbed on the metal surface [40-42]. Corrosion inhibition is initiated by the displacement of adsorbed water molecules by the bioactive species presented specially in the phenolic fraction such as hydroxytyrosol and tyrosol, leading to specific adsorption on the metal surface [43].
Thermodynamic parameters
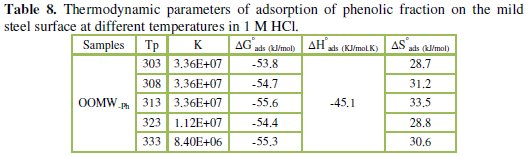
To calculate activation thermodynamic parameters of the corrosion reaction at various temperatures (303-333 K) in the presence and absence of various concentrations of the phenolic and non-phenolic fractions of olive oil mill wastewaters at 1 h of immersion, such as the energy Ea, the entropy ΔS0ads and the enthalpy ΔH0ads of activation, Arrhenius Eq. (7-8) and its alternative formulation called transition state Eq. (9-10) were used [44-45].


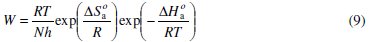
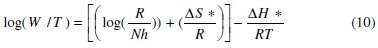
where T is the absolute temperature, K is a constant, Ea is the apparent activation corrosion energy, R is the universal gas constant, h is Plank's constant, N is Avogadro's number, ΔS0a is the entropy of activation and ΔH0a is the enthalpy of activation.
Fig. 3-6 show the Arrhenius plots for mild steel corrosion in 1 M HCl in the absence and presence of different concentrations of phenolic and non-phenolic fractions.
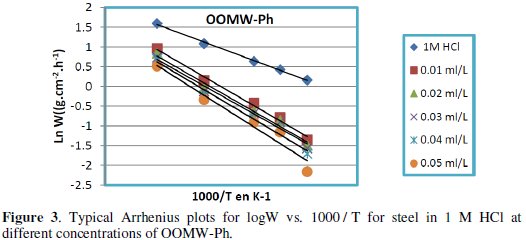
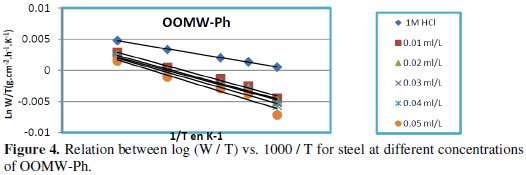
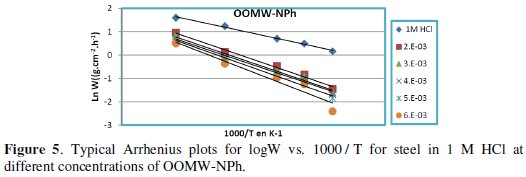
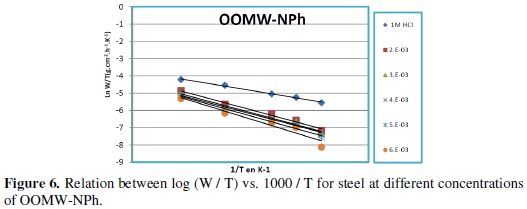
The activation energy Ea is calculated from the slope of the plots of Ln(Wcorr) vs. 1/T [Fig. 3 and 5] for both phenolic and non-phenolic fractions. Plots of Ln(Wcorr/T) vs. 1/T give a straight line with a slope of ΔH0/R and an intercept of (Log(R/Nh) + ΔS0/R), as shown in [Fig. 4 and 6]; the values of Ea, ΔH0 and ΔS0 are listed in Table 7.
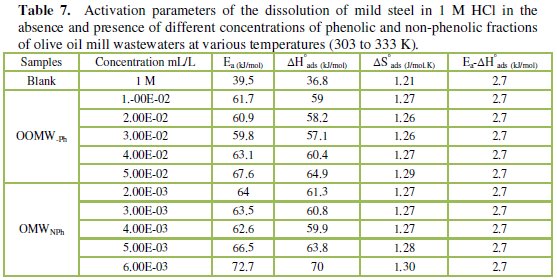
Inspection of the data shows that the activation energy is higher in the presence of both phenolic and non-phenolic fractions of the olive oil mill wastewaters than in its absence; the increase in Ea with phenolic and non-phenolic fractions concentration as shown in Table 7 is typical of a physisorption mechanism [46]. The decrease of the OOMW-Ph and OOMW-NPh efficiencies with temperature rise leading to a higher value of Ea, when compared to that in an uninhibited acid, is interpreted as an indication for an electrostatic character of the inhibitor's adsorption [47]. But Ea variation is not the unique parameter to affirm such mode of adsorption; free adsorption enthalpy ΔG0ads and enthalpy ΔH0ads, must also be considered. The positive values of ΔH0ads both in the absence and presence of additives of both phenolic and non-phenolic fractions indicate the endothermic nature of the activation process [48].
It is also seen in the tables that Ea and ΔH0ads vary in the same manner; however, the values of ΔH0ads are lower than those of Ea. Also, the entropy ΔS0 increases more positively with the presence of the inhibitor than in the presence of the non- inhibited one. This reflects the formation of an ordered stable layer of the inhibitor on the steel surface [49]. From the previous data, we can conclude that OOMW-Ph is an effective inhibitor. This phenomenon is often interpreted with physical character and formation of an adsorption film of electrostatic character [50].
Adsorption isotherm
The adsorption isotherms are usually used to describe the adsorption process, which is dependent on electronic properties of the inhibitor, the nature of the metal surface, the temperature, and the steric effects of different degrees site activity [51]. The establishment of adsorption isotherms that describe the adsorption of a corrosion inhibitor can provide important information to the nature of the metal-inhibitor interaction. Adsorption of the phenolic fraction occurs as the interaction energy between molecule and metal surface is higher than that between the water molecule and the metal surface [52].
In order to obtain the adsorption isotherm, the degree of surface coverage (Θ) for various concentrations of the phenolic fraction has been calculated at a temperature range from 303 to 333 K from the weight loss measurements. The results obtained for OOMW-Ph in 1 M HCl solution fit well Langmuir adsorption isotherm given by Eq. (11) [53-54]:

where θ is the degree of surface coverage, Cinh is the inhibitor concentration in the electrolyte, and Kads is the equilibrium constant of the adsorption process and is related to the standard Gibbs energy of adsorption, ΔG0ads, according to Eqs. (12-15) [55]:
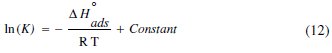



where R is the universal gas constant and T is the absolute temperature; the value 55.5 in the above equation is the concentration of water in solution in mol/L. The plot of Cinh/θ vs. Cinh gave a straight line with an intercept of 1 K-1; the Langmuir adsorption isotherms for the adsorption of inhibitor (OOMW-Ph) on the steel surface are shown in Fig. 7.
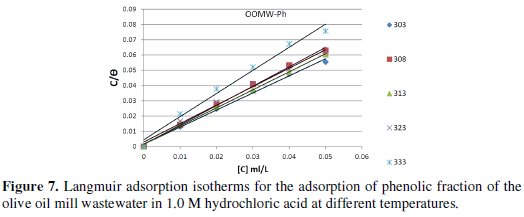
The strong correlation shows that the phenolic fraction tested was adsorbed on the steel surface electrode according to the Langmuir isotherm [56]. The values of ΔGads are all negative indicating that the phenolic fraction is strongly adsorbed on the steel surface [57]. The negative values of ΔGads indicate that the adsorption is spontaneous [58]. When the absolute value of ΔGads is higher than 40 kJ /mol, the adsorption could be seen as chemisorption. In this process, the covalent bond is formed by the charge sharing or transferring from the inhibitor molecules to the metal surface [59-60]; the calculated K values are shown in Table 8, suggesting the formation for the phenolic fraction of a chemisorbed film on the surface of the steel [61]; the phenolic fraction is adsorbed on the surface of steel according to Langmuir isotherm with interaction between the adsorbed molecules.
Comparison of the inhibition efficiencies obtained by gravimetric method for the two phenolic and non-phenolic fractions (88.9 %) OOMW-Ph and OOMW-NPh (89.1 %) reveals that we assist really to an intermolecular synergistic effect. In addition to the principal active constituent, the extracts contain other heterocyclic compounds as well. Therefore it is recommended not to determine ΔGads values in such situations [62].
Non-phenolic fraction (OOMW-NPh)
From the results of this research, it should be noted that an extract of olive oil mill wastewaters is complex and therefore it is always higher than the pure phenolic compound. Non-identified compounds as other phenolic compounds, proteins, amino acids, and sugars, are present in substantial quantities of low potential of the reinforcing heavier molecules such as hydroxytyrosol. Anticorrosive compounds, whatever their content, mode in a reaction cell (olive fruit) in olive oil by-product mill wastewaters, do not act independently; they interact in redox equilibria involving regeneration of new compounds with very large quantities in the case of hydroxytyrosol whatsoever coming from the hydrolysis of oleuropein or secoiridoid derivatives.
Phenolic fraction (OOMW-Ph)
The most abundant phenol in olives is oleuropein, formed from elenolic acid, glucose and hydroxytyrosol [31; 63-67]. During the olive ripening, oleuropein decreases, while its free components increase. In the ripe olive, hydroxytyrosol is present in quantities ranging from 1 to 3 g per 100 g-1 of dried weight [68-70] while oleuropein is fully transformed. In the extract OMWW oleuropein was present only in traces. This fact was foreseeable, as a very small amount of oleuropein is generally present in olive mill wastewaters, because enzymatic reactions degrade during olive ripening or processing [67]; the most abundant phenolic compound present in the OMWW extracts resulted to be hydroxytyrosol, which is formed as a result both of hydrolysis of oleuropein during oil extraction [71] and of acid hydrolysis of secoiridoid derivatives caused by the addition of HCl to the OMWW. In general, [72] the major phenolic compounds of olive oil mill wastewater contain hydroxytyrosol (70.93%) and tyrosol (16.55%), and the minor one contains, respectively, homovanillyl alcohol, protocatechuic acid, caffeic acid, 4-hydroxybenzoic acid, vanillic acid and 3,4-dihydroxyphenylglycol. Similar composition from OMW was determined by several authors [35; 67; 73].
From one liter of OMWW it is possible to obtain an extract containing 1.2 g of hydroxytyrosol and about 0.4 g of flavonoids, which in turn can be fractionated with the consequent production of 1 g of purified hydroxytyrosol (87% of the total amount in the extract) from one liter of OMWW [35].
Fig. 8 shows the most bioactive of phenolic content presented in the olive oil mill wastewaters..
Conclusions
From the overall experimental results the following conclusions can be deduced: The phenolic and non-phenolic fractions of the olive oil mill wastewaters are efficient inhibitors for the corrosion of steel in 1 M HCl.
The inhibition efficiency of both OOMW-Ph and OOMW-NPh increases with the concentration to attain a maximum value of 88.9% at the concentration 0.05 mL/L, and 89.1% at the concentration of 0.006 mL/L of phenolic and non- phenolic fractions, respectively.
OOMW-Ph and OOMW-NPh act as cathodic inhibitors by modifying the hydrogen reduction mechanism.
The inhibition efficiency of both fractions decreases with the rise of temperature. The perfect valorisation of olive oil extraction products (Olive Oil Mill Wastewaters) requires treatment of the extracted fraction.
References
1. Landolt D. Presse Polytechniques et Universitaire Ramandes. 1993;12:20. [ Links ]
2. Hausler R. Corrosion Chemistry. ACS Symp Series, Am Chem Soc. 1979; 262. [ Links ]
3. Ivanov ES. Metallurgiia, Moscow. 1986;175. [ Links ]
4. Ammar IA, El Khorafi FM. WerkstsfKorros. 1973; 24:702. [ Links ]
5. Abed Y, Hammouti B, Taleb M, et al. Trans SAEST. 2002;37:92. [ Links ]
6. Abed Y, Kissi M, Hammouti B, et al. Prog Org Coat. 2004; 50: 144. [ Links ]
7. Putilova LN, Balezin SA, Barrinik VP. New York: Pergamon Press; 1960. [ Links ]
8. Tan YJ, Bailey S, Kinsella BB. Corros Sci. 1996;38:1545. [ Links ]
9. Jovancicevic V, Ramachandran S, Prince P. Corrosion. 1999;55:449. [ Links ]
10. Bentiss F, Triasnel M, Vezin H, et al. Corros Sci. 2003;45:371. [ Links ]
11. Saji VS. Recent Patents on Corrosion Science. 2010;2:6. [ Links ]
12. Sangeetha M, Rajendran S, Muthumegala TS, et al. Zastita Materijala. 2011;52:3. [ Links ]
13. Broussard G, Bramantit O, Marchese FM. Occup Med. 1997;47:337. [ Links ]
14. Eddy NO, Ebenso EE. Afr J Pure Appl Chem. 2008;2:46. [ Links ]
15. Raja PB, Sethuraman MG. Mater Lett. 2008;62:113. [ Links ]
16. Bouknana D, Hammouti B, Bouyenzer A, et al. J Chem Pharm Res. 2013,5:1179. [ Links ]
17. Obied HK, Allen MS, Bedgood DR, et al. J Agric Food Chem. 2005;53:823. [ Links ]
18. Hamdi M. Appl Biochem Biotech. 1992;37:155. [ Links ]
19. Caruso D, Colombo R, Patelli R, et al. J Agric Food Chem. 2000;48:1182. [ Links ]
20. Obied HK, Bedgood Jr, Prenzler PD, et al. Anal Chim Acta. 2007;603:176. [ Links ]
21. Artajo LS, Romero MP, Motilva MJ. J Sci Food Agric. 2006;86:518. [ Links ]
22. Mulinacci N, Romani A, Galardi C, et al. J Agric Food Chem. 2001;49:3509. [ Links ]
23. El Hadrami A, Belaqziz M, El Hassni M, et al. J Agron. 2004;3:247. [ Links ]
24. Bianco A, Buiarelli F, Cartoni G, et al. J Sep Sci. 2003;26:417. [ Links ]
25. Sayadi S, Allouche N, Jaoka M, et al. Process Biochem. 2000;35:725. [ Links ]
26. Lesage-Meesen L, Navarro D, Maunier S, et al. Food Chem. 2001;75:501. [ Links ]
27. Esti M, Cinquanta L, La Notte E. J Agric Food Chem. 1998;46:32. [ Links ]
28. Romani A, Mulinacci N, Pinelli P, et al. J Agric Food Chem. 1999;47:946. [ Links ]
29. Savarese M, De Marco E, Sacchi R. Food Chem. 2007;105:761. [ Links ]
30. Rodis PS, Karathanos VT, Mantzavinou A. J Agric Food Chem. 2002;50:596. [ Links ]
31. Tuck KL, Hayball PJ. J Nutr Biochem. 2002;13:636. [ Links ]
32. De Leonardis A, Macciola V, Lembo G, et al. Food Chem. 2007;100:998. [ Links ]
33. Di Giovacchino L, Sestili S, Di Vincenzo D. Eur J Lipid Sci Tech. 2002;104:587. [ Links ]
34. Niaounakis M, Halvadakis CP. In Olive-mill waste management, Literature review and patent survey.Atene: Typothito-George Dardanos waste management serie 5. 2004.
35. De Marco E, Savarese M, Paduano A, et al. Food Chem. 2007;104:858. [ Links ]
36. Falleh H, Ksouri R, Chaieb K, et al. Compt Rend Biol. 2008;331:372. [ Links ]
37. Ryan D, Robardo K, Lavee S. Int J Food Sci Tech. 1999;34:265. [ Links ]
38. Elachouri M, Infante MR, Izquerdo F, et al. Corros Sci. 2001;43:19. [ Links ]
39. Rosliza R, Wan Nik WB, Senin H B. Mater Chem Phys. 2008;107:281. [ Links ]
40. Oguzie E E , Onuchukwu A I. Corros Rev. 2007;25:355. [ Links ]
41. Afia L, Salghi R, Bammou L, et al. J Saudi Chem Soc. 2011. [ Links ]
42. Abboud Y, Abourriche, Saffaj T, et al. Desalination. 2009;237:175. [ Links ]
43. Solmaz R, Kardas G, Yazici B, et al. Colloids Surf. A: Physicochem Eng Asp. 2008;312:7. [ Links ]
44. O'M Bockris J, Reddy A K N. Modern Electrochemistry. New York: Plenum Press; 1977. Vol 2. [ Links ]
45. Gadallah A G, Abd El-Rahman H A, AbouRomia M M. J Appl Electrochem. 1988;18:532. [ Links ]
46. Obot I B, Obi-Egbedi N O, Eseola A O. Ind Eng Chem Res. 2011;50:2098. [ Links ]
47. A Popova, Corros Sci. 2007;49:2144.
48. Bouklah M, Hammouti B, Lagrenee M, et al. Corros Sci. 2006;48:2831. [ Links ]
49. Yurt A, Balaban A, Kandemir S U, et al. Mater Chem Phys. 2004;85:420. [ Links ]
50. Popova A, Sokolova E, Raicheva S, et al. Corros Sci. 2003;45:33. [ Links ]
51. Yaunyuan F, Shenhao C, Wenjuan G, et al. J Electroanal Chem. 2007;602:115. [ Links ]
52. Moretti G, Quartarone G, Tassan A, et al. Mater Corros. 1994;45:641. [ Links ]
53. Doner A, Solmaz R, Ozcan M, et al. Corros Sci. 2011;53:2902. [ Links ]
54. Sanatkumar B S, Nayak J, Shetty A N. J Coat Technol Res. 2011;4:1. [ Links ]
55. Elayyachy M, El Idrissi A, Hammouti B. Corros Sci. 2006;48:2470. [ Links ]
56. Mora N, Cano E, Polo J L, et al. Corros Sci. 2004;46:563. [ Links ]
57. Talati J D, Gandhi D K. Corros Sci. 1983;23:1315. [ Links ]
58. Chu A K P, Sukawa A J. J Electrochem Soc. 1969;116:1188. [ Links ]
59. Szlarska-Smialowska Z. Corros Sci. 1978;18:953. [ Links ]
60. Yurt A, Ulutas S, Dal H. Appl Surf Sci. 2006;253:919. [ Links ]
61. Villamil R F V, Corio P, Rubin J C, et al. J Electroanal Chem. 2002;535:75. [ Links ]
62. Benali O, Benmehdi H, Hasnaoui O, et al. J Mater Environ Sci. 2013;4:127. [ Links ]
63. Gariboldi P, Jommi G, Verrotta L. Phytochemistry. 1986;25:865. [ Links ]
64. Panizzi L, Scarpati M L, Oriente E G. Gazz Chim Ital.1960;90:1449. [ Links ]
65. Servili M, Baldioli M, Selvaggini R, et al. J Am Oil Chem Soc. 1999;76:873. [ Links ]
66. Soler-Rivas C, Espin J C, Wichers H J. J Sci Food Agric. 2000;80:1013. [ Links ]
67. De Leonardis A, Macciola V, Lembo G, et al. Food Chem. 2007;100:998. [ Links ]
68. Amiot M T, Fleuriet A, Macheix J T. J Agric Food Chem. 1986;34:823. [ Links ]
69. Amiot M T, Fleuriet A, Macheix J T. Photochemistry. 1989;28:67. [ Links ]
70. Romero C, Brenes M, Garcia P, et al. J Agric Food Chem. 2002;50:3835. [ Links ]
71. Capasso R, Evidente A, Visca C. Agrochimica. 1994;38:165. [ Links ]
72. Kallel M, Belaid C, Mechichi T, et al. Chem Eng J. 2009;150: 391-395. [ Links ]
73. Khoufi S, Aloui F, Sayadi S. J Hazard Mater. 2008;151:531. [ Links ]
Acknowledgements
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-VPP-089.
*Corresponding author. E-mail address: hammoutib@gmail.com
Received 4 February 2014; accepted 27 February 2014














