Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.32 no.4 Coimbra jul. 2014
https://doi.org/10.4152/pea.201404253
Electrochemical Properties of (η5-C5Me5)-Rhodium and - Iridium Complexes Containing Bis(pyrazolyl)alkane Ligands
Luisa M.D.R.S. Martinsa,b,* , Claudio Pettinaric , Fabio Marchettid , Elisabete C.B.A. Alegriaa,b and Armando J.L. Pombeirob
a Chemical Engineering Department, ISEL, R. Conselheiro Emídio Navarro, 1959-007 Lisboa, Portugal
b Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001 Lisboa, Portugal
c School of Pharmacy, University of Camerino, S. Agostino 1, 62032 Camerino, Italy
d School of Science and Technology, University of Camerino, S. Agostino 1, 62032 Camerino, Italy
Abstract
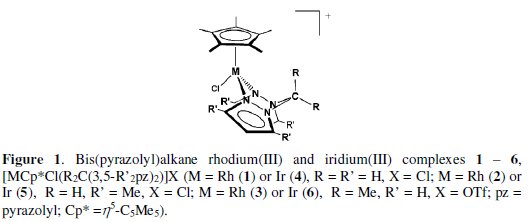
The electrochemical properties of rhodium(III) 1-3 and iridium(III) 4-6 complexes containing bis(pyrazolyl)alkane ligands [MCp*Cl(R2C(3,5-R'2pz)2)]X (M = Rh (1) or Ir (4), R = R' = H, X = Cl; M = Rh (2) or Ir (5), R=H,R'=Me,X=Cl;M=Rh(3) or Ir (6), R=Me,R'=H,X=OTf;pz=pyrazolyl;Cp*=η5-C5Me5) were investigated by cyclic voltammetry and controlled potential electrolysis. They exhibit two sequential irreversible reductions assigned to the MIII → MII and MII → MI reductions, which are dependent on the methylation of the bis(pyrazolyl)alkane ligands.
Keywords: bis(pyrazolyl)alkane, rhodium(III) and iridium(III) complexes, cyclic voltammetry, controlled potential electrolysis.
Introduction
Poly(pyrazolyl)borates and their metal complexes are widely studied and applied in several areas of chemistry [1,2]. We have been interested [3-10] in the coordination chemistry of their much less studied carbon analogues, the poly(pyrazolyl)alkane-type scorpionates, as well as on the synthesis of new derivatives [11-13] either by substitution at the pyrazolyl rings or functionalization of the apical carbon, which can play a critical role for the modification of their properties.
The knowledge that Rh(I) [14] and Ir(I) [15] compounds containing dihydro(pyrazolyl)methane [H2C(3,5-R2pz)2, (R = H or Me; pz = pyrazolyl)] ligands exhibit interesting applications namely in catalysis, prompted us [9] to undertake a systematic study of the reactions between [MCp*Cl(μ-Cl)]2 dimers (M = Rh or Ir; Cp* = η5-C5Me5) and bis(pyrazolyl)alkanes R2C(pzR'2)2 (R, R' = H or Me). Rhodium(III) and iridium(III) complexes of general formula [MCp*Cl(R2C(3,5-R'2pz)2)]X (M = Rh or Ir; R, R' = H or Me; X = Cl or OTf) have been prepared (Fig. 1). Herein we report their electrochemical properties using cyclic voltammetry (CV) and controlled potential electrolysis (CPE) techniques.
Experimental
The electrochemical experiments were performed on an EG&G PAR 273A potentiostat/galvanostat connected to a personal computer through a GPIB interface. Cyclic voltammograms were obtained in 0.2 M [nBu4N][BF4]/CH2Cl2, at a platinum disc-working electrode (d = 0.5 mm) and at 25 °C. Controlled- potential electrolyses were carried out in electrolyte solutions with the above- mentioned composition, in a three-electrode H-type cell. The compartments were separated by a sintered glass frit and equipped with platinum gauze working and counter electrodes. For both CV and CPE experiments, a Luggin capillary connected to a silver wire pseudo-reference electrode was used to control the working electrode potential, and a Pt wire was employed as the counter-electrode for the CV cell. The CPE experiments were monitored regularly by cyclic voltammetry, thus assuring no significant potential drift occurred along the electrolyses. The redox potentials of the complexes were measured by CV in the presence of ferrocene as the internal standard, and their values are quoted relative to the SCE by using the [Fe(η5-C5H5)2]0/+ redox couple (E1/2ox = 0.525 V vs. SCE) [21,22].
Results and discussion
The redox properties of bis(pyrazolyl)alkane rhodium(III) and iridium(III) compounds [MCp*Cl(R2C(3,5-R'2pz)2)]X (M = Rh (1) or Ir (4), R = R' = H, X = Cl; M = Rh (2) or Ir (5), R=H,R'=Me,X=Cl;M=Rh(3) or Ir (6), R=Me, R' = H, X = OTf) have been investigated by cyclic voltammetry, at a Pt electrode, in a 0.2 M [nBu4N][BF4]/CH2Cl2 solution, at 25 °C. They exhibit (Fig. 2 for complex 3) two single-electron irreversible reduction waves, assigned to the MIII → MII (wave I) and MII → MI (wave II) reductions, at the reduction peak potential values given in Table 1 (IEpred in the range from -0.88 to -1.28 V vs. SCE, and IIEpred between -1.27 and -1.80 V vs. SCE).
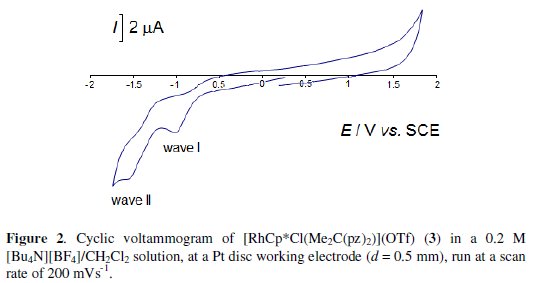
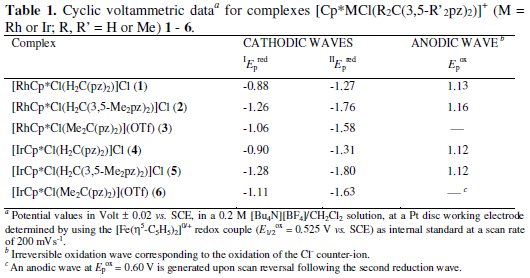
The occurrence of a single-electron reduction per MIII (or MII) atom has been confirmed by exhaustive controlled potential electrolysis (CPE) at a potential slightly cathodic to that of the peak potentials of wave I (or II). CPE at any of the reduction waves corresponds to a charge consumption of 1 F/mole of complex.
In addition, compounds 1, 2, 4 and 5 exhibit a single-electron (CPE) irreversible oxidation wave, at Epox ca. 1.1 V vs. SCE, corresponding to the oxidation of the Cl- counter-ion. No other oxidation wave has been detected, for any of the complexes, by a first anodic sweep without a previous reduction scan, indicating that neither a metal centred nor a ligand-centred oxidation are observed.
The values of the MIII → MII (wave I) and MII → MI (wave II) reduction potentials of 1 -3 and 4 -6 reflect the electron-donor character of their ligands as follows. Methylation at the apical carbon (R = Me) of the dihydrobis(pyrazolyl)methane ligand results in a measurable (ca. 0.3 V) cathodic shift of the potential, in accord with the better electron-donor character of the Me2C(pz)2 ligand in comparison with H2C(pz)2. An even more pronounced effect (cathodic shift of ca. 0.4 -0.5 V) is observed if the hydrogen replacement by methyl occurs at the pyrazolyl rings (R' = Me) of the dihydrobis(pyrazolyl)methane ligand. This is consistent with the shorter distance of the latter electron-donor groups to the metal compared with the previous case when they are bound to the apical carbon. However, any analysis has to be taken rather cautiously in view of the irreversible character of the oxidation waves.
This study could provide an opportunity to estimate the EL ligand parameter for the new bis(pyrazolyl)alkane ligands (although with the above limitation) by applying the Lever equation (1) [16,17], which relates linearly the redox potential (E in V vs. the standard hydrogen electrode (SHE)) of an octahedral complex with the sum (SEL) of the EL ligand parameters for all the ligands (2-electron donors, assuming additive contributions), and assuming that equation (1), valid for half-sandwich arene-type complexes [8,18-20], is also valid for half- sandwich Cp-type complexes.
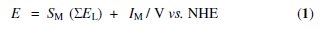
However, the slope (SM) and the intercept (IM), which are dependent upon the metal, redox couple, spin state and stereochemistry [16,17], are not know for the present redox couples and therefore the determination of the EL ligand parameter was precluded.
Conclusions
This electrochemical study has allowed to determine the redox potentials of bis(pyrazolyl)alkane rhodium(III) and iridium(III) compounds bearing the Cp* ligand. Moreover, it allowed to compare the electron-donor characters of different methylated bis(pyrazolyl)alkane ligands, although one should be rather cautious due to the irreversibility of the reduction waves. The quantification of that effect by the Lever formalism (by using equation 1) was not possible since the SM and IM values for the present redox couples are not known.
References
1. Trofimenko S. In: Scorpionates, The Coordination Chemistry of Polypyrazolylborate Ligands. London: Imperial College Press; 1999. [ Links ]
2. Pettinari C. In: Scorpionates II: Chelating Borate Ligands. Imperial College Press, World Scientific Pub; 2008. [ Links ]
3. Martins LMDRS, Pombeiro AJL. Coord Chem Rev. 2014;265:74. [ Links ]
4. Dinoi C, Guedes da Silva MFC, Alegria E, et al. Eur J Inorg Chem. 2010;2415. [ Links ]
5. Rocha BGM, Wanke R, Guedes da Silva MFC, et al. J Organomet Chem. 2012; 714. [ Links ]
6. Peixoto de Almeida M, Martins LMDRS, Carabineiro SAC, et al. Catal Sci Technol. 2013;3:3056. [ Links ]
7. Pettinari C, Marchetti F, Lupidi G, et al. Inorg Chem. 2011;50:11173. [ Links ]
8. Pettinari C, Marchetti F, Cerquetella A, et al. Organometallics 2011;30:1616. [ Links ]
9. Pettinari C, Pettinari R, Marchetti F, et al. Inorg Chem. 2007;46:896. [ Links ]
10. Carmona E, Cingolani A, Marchetti F, et al. Organometallics. 2003;22:2820. [ Links ]
11. Wanke R, Smolenski P, Guedes da Silva MFC, et al. Inorg Chem. 2008;47:10158. [ Links ]
12. Wanke R, Guedes da Silva MFC, Lancianesi S, et al. Inorg Chem. 2010;49:7941. [ Links ]
13. Silva TFS, Mishra GS, Guedes da Silva MFC, et al. Dalton Trans. 2009;81:1217. [ Links ]
14. Teuma E, Loy M, Le Berre C, et al. Organometallics. 2003;22:5261. [ Links ]
15. Field LD, Messerle BA, Rehr M, et al. Organometallics. 2003;22:2387. [ Links ]
16. Lever ABP. Inorg Chem. 1990;29:1271. [ Links ]
17. Lever ABP. In: Lever ABP, editor. Comprehensive coordination chemistry II. Vol. 2. Oxford: Elsevier; 2004. Chapter 2.19; p.251-68; and references therein. [ Links ]
18. Guedes da Silva MFC, Pombeiro AJL. Electrochim Acta. 2012;82:478. [ Links ]
19. Marchetti F, Pettinari C, Cerquetella A, et al. Inorg. Chem. 2009;48:6096. [ Links ]v
20. Marchetti F, Pettinari C, Pettinari R, et al. Organometallics. 2011;30:6180. [ Links ]
21. Pombeiro AJL, Guedes da Silva MFC, Lemos Manada. Coord. Chem. Rev. 219 (2001) 53.
22. Silva Menpra, Pombeiro AJL, Frausto da Silva JJR, et al. J Organometal Chem. 1991;421:75.
Acknowledgements
This work has been partially supported by the Fundação para a Ciência e a Tecnologia (FCT), Portugal, and projects PTDC/QUI-QUI/102150/2008, PTDC/EQUEQU/ 122025-2010 and Pest-OE/QUI/UI0100/2013.
*Corresponding author. E-mail address: lmartins@deq.isel.ipl.pt
Received 1 July 2014; accepted 23 August 2014














