Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.34 no.6 Coimbra nov. 2016
https://doi.org/10.4152/pea.201606395
Aromatic Carboxylic Acids as Corrosion Inhibitors for Aluminium in Alkaline Solution
Ali Reza Madram* , Foroozan Shokri , Mohammad Reza Sovizi and Hamide Kalhor
Department of Chemistry and Chemical Engineering, Malek Ashtar University of Technology, Tehran 15875-1774, I.R. Iran
Abstract
The corrosion behavior of aluminium in 1 M NaOH solution, in the absence and presence of some aromatic carboxylic acids, was investigated using potentiodynamic polarization techniques, electrochemical impedance spectroscopy (EIS) and scanning electron microscope (SEM). The results among the investigated aromatic carboxylic acids, 4-bromomethyl, 3-bromo and 3-hydroxy benzoic acid were more efficient corrosion inhibitors for aluminium in alkaline medium. The values of different thermodynamic parameters such as adsorption equilibrium constant (Kads) and free energy of adsorption (ΔGads) were calculated and discussed. The adsorption process of studied inhibitors on aluminium surface obeys the Langmuir adsorption isotherm.
Keywords: aluminium corrosion, aromatic carboxylic acids, inhibitor, electrochemical impedance spectroscopy, potentiodynamic polarization.
Introduction
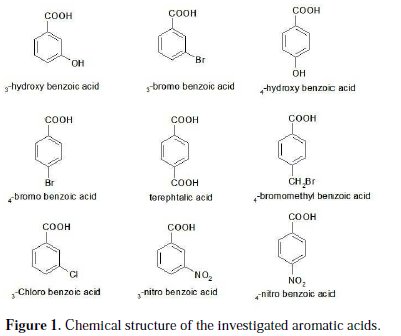
Aluminium is an attractive fuel for electrochemical power sources. Its principal advantages as an anode material include high power, energy density, and great abundance. Corrosion of aluminium and its alloys has been the subject of numerous studies, due to their wide range of industrial applications, especially in aerospace, surface coating and alkaline batteries [1-3]. Aluminium and its alloys, however, are reactive materials and are prone to corrode. Aluminium relies on the formation of a compact, adherent passive oxide film for its corrosion immunity in various environments. This surface film is amphoteric and dissolves substantially when the metal is exposed to high concentrations of acids or bases [4]. Alkali destroys the protective aluminium film very quickly, because OH- ions are adsorbed, thus increasing the dissolution rate of aluminium [5]. The corrosion inhibition of aluminium and its alloys is of tremendous technological importance, due to the increased applications of these materials. Many investigators have studied ways to obtain optimum corrosion protection for aluminium in various media, by either finding new inhibitors or improving the inhibition efficiency. The inhibitor efficiency has been attributed to the adsorption of the inhibitor molecules on the metal surface, forming a protective layer. The extent of adsorption of an inhibitor depends on many factors: (a) the nature of the metal, (b) the mode of adsorption of the inhibitor, and (c) the surface condition [6-8]. The inhibition effect mainly depends on some physicochemical and electronic properties of the organic inhibitor which relate to its functional groups, steric effects, electronic density of donor atoms, and orbital character of donating electrons [9-12]. Several inhibitors have been used to control the corrosion of aluminium. To prevent the corrosion of aluminium in acid medium, inhibitors such as antibacterial drugs [13], imidazoline derivatives [14], Schiff bases [15] and delonix regia extract [16] have been used. In alkaline medium, inhibitors such as polyvinyl alcohol [17], gongronema latifolium extract [18] and 3-hydroxyflavone [19] have been used to prevent corrosion of aluminium. Aromatic carboxylic acid, especially benzoic acids and their substituted compounds were investigated as effective inhibitors for aluminium in acidic and alkaline solutions [20]. Adsorptive characteristics of the benzoic acids have an influence on the corrosion inhibition capacities. The benzene ring attached to the carboxyl group contributes to the inhibitor efficiency, which is further influenced by the substituent in the benzene ring. Thus, the present study was undertaken to compare the inhibitive abilities of some aromatic carboxylic acids shown in Fig. 1 on the corrosion of aluminium in 1 M NaOH solutions by using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) methods.
The surface analysis was carried out using scanning electron microscope (SEM). The adsorption behavior of the inhibitors was analyzed against Langmuir adsorption isotherm theory.
Experimental
Materials and methods
The working electrode was made of aluminium (Al-1050); surface preparation consisted of abrasion with sandpaper (P 800-2000, Siawat®), followed by degreasing in an ultrasonic bath using ethanol and deionized water, and drying with a stream of air. The investigated aromatic carboxylic acids (Fig. 1) were of analytical grade and used as purchased without further purification.
Electrochemical experiments
The electrochemical experiments were carried out using a traditional three- electrode cell. The working electrode was an aluminium electrode (0.5 cm2). The counter electrode was a platinum foil and the reference electrode was an Hg/HgO electrode. Electrochemical measurements were performed by a Bio Logic (SP-150) instrument. Impedance measurements were performed under the corrosion potential with a sinusoidal potential perturbation of 5 mV in amplitude, and the frequency was from 100 kHz to 10 mHz. The measurements were taken until the electrode reached a steady state.
Results and discussion
Polarization measurements
Polarization measurements were performed in the absence (blank solution) and presence of various concentrations (100, 250 and 500 ppm) of the investigated aromatic carboxylic acids in 1 M NaOH at 25 °C. Fig. 2 shows typical Tafel polarization curves for aluminium in 1 M NaOH for blank solution, and in the presence of the more efficient investigated aromatic carboxylic acids (3-bromo, 4bromomethyl and 3-hydroxy benzoic acid), at a concentration of 500 μg/mL and a sweep rate of 0.5 mV s-1 at 25 °C.
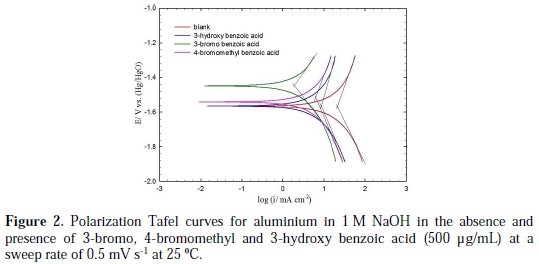
The data clearly show that the addition of the derivatives of benzoic acids shifts the corrosion potential (Ecorr) in the positive direction (especially for the 3-bromo and 4-bromomethyl derivatives) and reduces both the anodic and cathodic current densities. The anodic current was, however, reduced more significantly than the cathodic current for 3-bromo, 4-bromomethyl and 3-hydroxy derivatives of benzoic acid (Fig. 2).
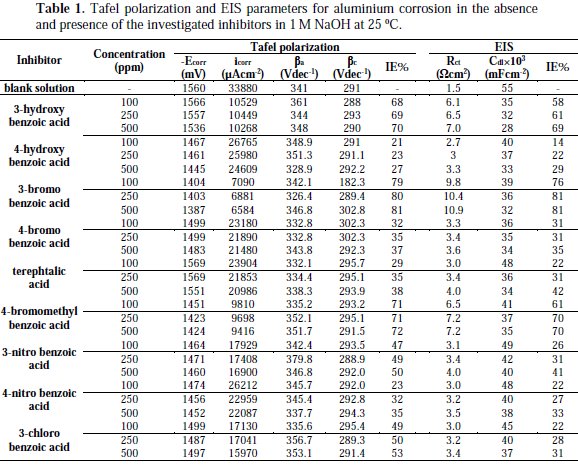
These results indicate that these three aromatic carboxylic acids act as mixed-type inhibitors. This means that these inhibitors have significant effects on retarding the cathodic hydrogen evolution reaction and inhibiting the anodic dissolution of aluminium in alkaline solution. The Tafel extrapolation method was used to evaluate the corrosion parameters and the values of related electrochemical parameters, i.e. Ecorr, corrosion current density (icorr), and inhibition efficiency (IE%) were calculated from the related polarization curves, and are presented in Table 1.
The percentage inhibition efficiency (IE%) was calculated as follows [21]:
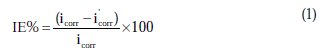
where icorr and i'corr are the corrosion current densities in the absence and presence of inhibitors, respectively. The values of cathodic (βc) and anodic (βa) Tafel constants were calculated from the linear region of the polarization curves. As presented in Table 1, the corrosion current density was reduced in the presence of all investigated aromatic carboxylic acids, especially in the presence of 3-bromo, 4-bromomethyl and 3-hydroxy benzoic acid. The values of IE% for these inhibitors at a concentration of 500 ppm were 81, 72 and 70%, respectively. Furthermore, the increase of inhibition efficiencies for the aluminium corrosion in 1 M NaOH, with increasing concentrations of organic acids, can be explained on the bases of the inhibitor adsorption.
This behavior can be attributed to the increased surface coverage θ, due to the increase of the number of adsorbed molecules at the metal surface [13]. So, the investigation of the relation between corrosion inhibition and adsorption is of great importance. The adsorption of organic inhibitors at the metal/solution interface takes place through the replacement of water molecules by organic inhibitor molecules, according to the following process [13]:
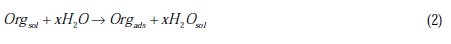
where Org(sol) and Org(ads) are organic molecules in the solution and adsorbed on the metal surface, respectively, and x is the number of water molecules replaced by the organic molecules. Different adsorption isotherms were analyzed in order to obtain more information about the interactions between aromatic carboxylic acids and the aluminium surface. The linear relationship between θ values and concentration of inhibitor, Cinh, is to be found in order to obtain the isotherm. The degree of θ is calculated from the polarization data using the following equation [13]:

Attempts were made to fit the θ values to various isotherms, including Langmuir, Temkin, Frumkin and Flory-Huggins. The best fit is obtained with the Langmuir isotherm. The Langmuir adsorption isotherm is given by [22]:

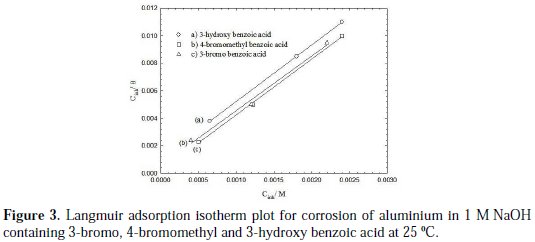
where Kads is the adsorption equilibrium constant. A plot of Cinh/θ against Cinh for the more efficient investigated inhibitors showed a straight line, indicating that adsorption follows the Langmuir adsorption isotherm, as shown in Fig. 3.
The values of Kads obtained from the Langmuir adsorption isotherm are related to the standard free energy of adsorption (ΔGads) [23]:
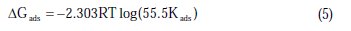
where R is the universal gas constant, T is the absolute temperature, and the value of 55.5 is the molar concentration of water in the solution. The values of Kads for the more efficient investigated inhibitors, i.e., 3-bromo, 4-bromomethyl and 3-hydroxy benzoic acid, were 1250, 1111 and 1000 L.mol-1, respectively. The higher value of Kads for 3-bromo benzoic acid indicates stronger adsorption on the aluminium surface. Furthermore, -ΔG°ads values for 3-bromo, 4-bromomethyl and 3-hydroxy benzoic acid were 27.6, 27.3 and 27.1 kJ.mol-1, respectively (Table 2).
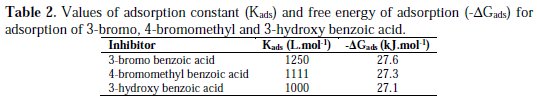
That indicates 3-bromo benzoic acid was more effectively adsorbed on aluminium surface than other investigated carboxylic acids. The negative values for ΔG°ads are consistent with the condition for spontaneity of the adsorption process and the stability of the adsorbed layer on the aluminium surface.
EIS measurements
The EIS were recorded for aluminium in the absence and presence of investigated aromatic carboxylic acids. The complex plane plots of the EIS measurements, obtained at open-circuit potential after one hour immersion in the absence and presence of 3-bromo, 4-bromomethyl and 3-hydroxy benzoic acid as the best investigated inhibitors, are presented in Fig. 4(a).
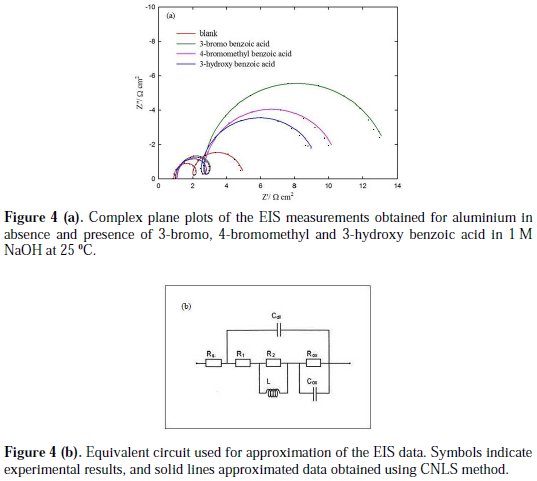
Furthermore, an equivalent circuit to describe the electrochemical impedance characteristics of aluminium is shown in Fig. 4(b).
It is composed of a Faradaic impedance parallel to a double layer capacitance: Cdl. Faradaic impedance consists of Rct (=R1+R2), indicating the charge transfer resistance at the metal/oxide film interface; L results from the interruption of anodic dissolution of aluminium by the surface charge build-up; Rox is the resistance against charge transport in the oxide film and Cox, due to the dielectric properties of surface oxide film. The Rs component in the circuit is a solution resistance with the value at a high frequency intercept on the real impedance axis [24]. This model circuit shows that the capacitive semicircles occurring at the high and low frequencies in Fig. 4(a) are due to the charge transfer reaction at the metal/surface oxide film, and the dielectric properties of surface oxide film, respectively. It has been reported that aluminium dissolves into the solution in the 3+ oxidation state through the generation of Al+ or Al+2 intermediate species [24]. Therefore, the charge transfer resistance, Rct, might be represented by the sum of R1 and R2 in the equivalent circuit. The inductive loop shown in the medium frequency range might have been caused by the intermediate species generated during metal dissolution. The EIS data obtained in the NaOH solution are shown in Table 1. Inspection of Table 1 reveals that Rct values increase prominently, while Cdl reduces with the concentration of all investigate inhibitors. Rct is associated with the corrosion resistance, so, the higher charge transfer resistance indicates better inhibition performance. The decrease in Cdl, compared with that in the blank solution, which can result from a decrease in local dielectric constant and/or an increase in the thickness of the electrical double layer, suggests that the inhibitor molecules function by adsorption at the solid/solution interface [25]. However, the values of the charge transfer and inhibition efficiency were increased in the presence of all inhibitors, especially in the presence of 3-bromo, 4-bromomethyl and 3-hydroxy derivatives of benzoic acid. These results are in good agreement with those obtained from polarization measurements (Fig. 2). The effect of temperature on the rate of dissolution of aluminium in 1 M NaOH containing 100 μg/mL of the 3-bromo benzoic acid, as the most efficient investigated inhibitor, was evaluated by the Tafel polarization and EIS methods over a temperature range from 25 to 80 °C (Table 3).
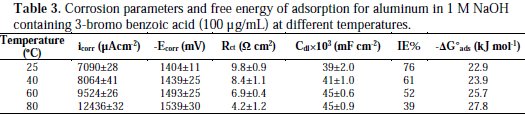
The results revealed that a higher temperature increased the corrosion rate of Al1050 for this inhibitor. Additionally, the increase in corrosion rate in the absence of 3-bromo benzoic acid was higher than in its presence at all temperatures, suggesting more aggressiveness of a free alkaline solution. It was proved that benzoic acid is a good corrosion inhibitor, probably because the dimeric structure is formed, and this structure is somehow preferable for adsorption [26]. The results of Table 3 revealed that the ΔG°ads values increased from -22.9 to -27.8 kJ mol-1 with increasing temperature from 25 to 80 °C; this means adsorption phenomena are affected by dimerization of 3-bromo benzoic acid on aluminium surface.
SEM studies
The SEM images of aluminium in 1 M NaOH solution in the absence (a) and presence (b) of 3-bromo benzoic acid (100 μg/mL) at 25 °C after 1 h exposure are given in Fig. 5.

The surface is porous and the large and deep holes appear. However, the appearance of Al surface is different after the addition of 3-boromo benzoic acid as the most investigated inhibitor for the corrosive solution. It can be seen from Fig. 5 that the dissolution rate of aluminium is considerably reduced, and the smooth surfaces appear by the formation of a protective film on the Al surface.
Conclusions
Some aromatic carboxylic acids were studied for the inhibition of aluminium corrosion in alkaline solution. The polarization and EIS measurements showed that 3-bromo, 4-bromomethyl and 3-hydroxy benzoic acid were the more effective inhibitors among all investigated aromatic carboxylic acids on corrosion of aluminium in 1 M NaOH at 25 °C. The IE% values calculated from polarization Tafel curves for these inhibitors at concentration of 100 ppm were: 79, 71 and 68, respectively. These results are in good agreement with those obtained from EIS measurements. Furthermore, the IE % was found to increase with an increase in inhibitors' concentration. The adsorption of these inhibitors on the aluminium surface obeyed the Langmuir adsorption isotherm model. The negative values of ΔG°ads indicate spontaneous adsorption of the inhibitors on the surface of aluminium. The effect of temperature on the rate of dissolution of aluminium in 1 M NaOH containing 100 μg/mL of the 3-bromo benzoic, as the most efficient investigated inhibitor, revealed that higher temperatures increased the corrosion rate of Al-1050 for this inhibitor. Surface morphological studies by SEM analysis were in good agreement with electrochemical measurements.
References
1. Hori Y, Takao J, Shomon H. Electrochim Acta. 1986;36:555. [ Links ]
2. Wilhelmsen W, Arnesen T, Hasvold O, et al. Electrochim Acta. 1991;36:79. [ Links ]
3. Zeinelabedin S, Saleh AO. J Appl Electrochem. 2004;34:331. [ Links ]
4. Ashassi-Sorkhabi H, Shabani B, Aligholipour B, et al. Appl Surf Sci. 2006;252:4039. [ Links ]
5. Noor AE. J Appl Electrochem. 2009;39:1465. [ Links ]
6. Zhang DQ, Gao LX, Zhou GD. Corros Sci. 2004;46:3031. [ Links ]
7. Elmorsi MA, Hassanein AM. Corros Sci. 1999;41:2337. [ Links ]
8. Curkovic HO, Stupnisek-Lisac E, Takenouti H. Corros Sci. 2010;52:398. [ Links ]
9. Lamaka SV, Zheludkevich ML, Yasakau KA, et al. Electrochim Acta. 2007;52:7231. [ Links ]
10. Branzoi V, Golgovici F, Branzoi F. Mater Chem Phys. 2003;78:122. [ Links ]
11. Fleischmann M, Hill IR, Mengoli G, et al. Electrochim Acta. 1985;30:879. [ Links ]
12. Sanja M, Metikos-Hukovic M. J Appl Electrochem. 2003;33:1137. [ Links ]
13. Abdallah M. Corros Sci. 2004;46:1981. [ Links ]
14. Quraishi MA, Rafique MZA, Khan S, et al. J Appl Electrochem. 2007;37:1153. [ Links ]
15. Safak S, Duran B, Yurt A, et al. Corros Sci. 2012;54:251. [ Links ]
16. Abiola OK, Oforka NC, Ebenso EE. et al. Anti-Corrosion Methods Mater. 2005;54:219. [ Links ]
17. Umoren SA, Ebens EE, Okafo PC, et al. J Appl Polym Sci. 2006;103:2810. [ Links ]
18. Oguzie EE, Onuoha GN, Ejike EN. Pigment Resin Technol. 2007;36:44. [ Links ]
19. Princey JM, Nagarajan P. J Korean Chem Soc. 2012;56:201. [ Links ]
20. Moussa MN, El-Tagoury MM, Radi AA, et al. Anti-Corros Methods Mater. 1990;37:4. [ Links ]
21. Tao Z, Zhang S, Li W, et al. Ind Eng Chem Res. 2011;50:6082. [ Links ]
22. Obot IB, Obi-Egbedi NO, Umoren SA. Corros Sci. 2009;51:1868. [ Links ]
23. Yurt A, Aykin O. Corros Sci. 2011;53:3725. [ Links ]
24. Lee KK, Kim KB. Corros Sci. 2001;43:561. [ Links ]
25. Lagrenee M, Mernari B, Bouanis M, et al. Corros Sci. 2002;44:573. [ Links ]
26. Bilgic S. Mater Chem Phys. 2002;76:52. [ Links ]
Acknowledgements
The authors are grateful to Malek-Ashtar University of Technology for providing the lab facilities to bring about this work.
*Corresponding author. E-mail address: ar.madram@gmail.com
Received March 05, 2016; accepted October 24, 2016














