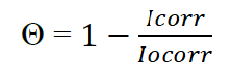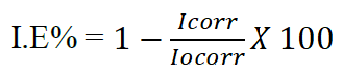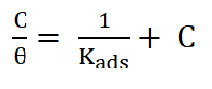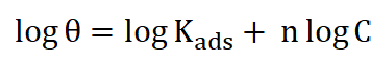Introduction
Corrosion is one of the most devastating occurrences in modern technology, and poses a serious threat to many industries. Corrosion is destructive to mild steel1 2 3. It is a crucial and costly problem in industries. It represents a considerably large proportion of loss, as a result of lost production, inefficient operation and high maintenance4,5. Hence, corrosion control is of paramount importance to scientists and engineers. The studies on steel corrosion phenomena have become an industrial and academic topic, especially on acidic and saline media, in recent years6 7 8. Several organic, as well as inorganic inhibitors, have been used to provide protection for metals, but quite often, these compounds can be potentially toxic, very costly, difficult to isolate and characterize from their natural sources9 10 11. For these reasons, recent researches have been directed towards inhibitive drugs. One of the most practical methods through which mild steel is protected against corrosion is the use of inhibitive drugs or organic inhibitors, which are becoming more and more popular, considering the recent studies12-16. The effectiveness of organic molecules is a function of their ability to adhere to the metal surface, which can distinctly alter the corrosion mitigating properties of mild steel17. This has assumed great significance, due to their application in preventing corrosion under different corrosive environments18-21. The use of drug inhibitors draws much attention, due to their high efficiency, easiness of synthesis and cost-effective nature. They show good corrosion protection and are highly environment-friendly22.
Drug inhibitors adsorption onto the steel surface forms a protective coating that restricts diffusion of chemical species involved in steel ionization. Numerous active drug components have been identified to be potent for mild steel protection, in different corrosive environments23,24. In most cases, expired drugs can be tested as corrosion inhibitors, and steel degrades only in an infinitesimal way25,26. This research aimed at examining the inhibitive ability of Cefalexin on mild steel corrosion in a seawater simulated environment (sodium chloride solution). The effect of Cefalexin, on the corrosion behaviour of mild steel, was observed using linear polarisation techniques, weight loss measurements and computational studies. Most antibiotics and their decomposition products possess electron donor groups that can bind naturally occurring metal ions, thereby forming complexes with them.
Experimental procedures
Sample preparation
The mild steel coupons used for these studies had the dimensions of 15 x 10 x 2 mm, with a 2 mm diameter hole drilled at the centre top of the samples. The spectrometer chemical composition in wt % is shown in Table 1.
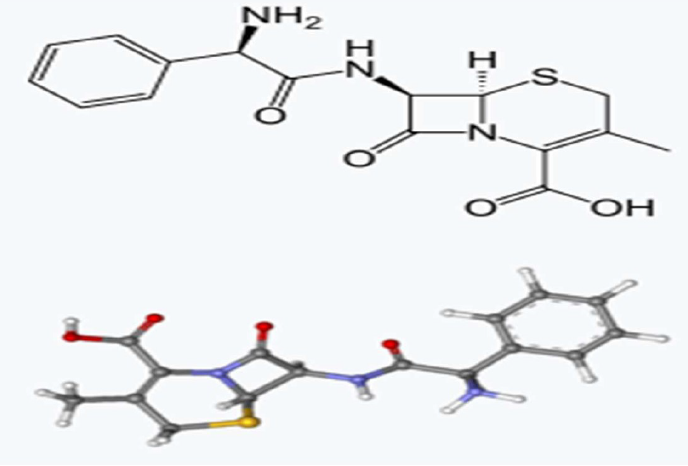
These coupons were then polished with emery papers of different grades, and each specimens weight was recorded and labelled appropriately. The mild steel specimens were placed in a sodium chloride (3.65% NaCl) solution of 200 mL, in plastic containers with inhibitors at concentrations of 0%, 10%, 30%, 50% and 100%, and they were monitored over a period of 21 days. The molecular structure of the prepared inhibitor is shown in Fig. 1, and its molecular mass is 347.389 g/mol. The electrochemical investigation was performed at ambient temperature.
Linear polarization Resistance
An Autolab PGSTAT 101 Metrohm potentiostat/galvanostat, connected to a computer, was used to obtain linear polarization measurements. The mild steel coupon was welded to a wire and mounted on a resin. Steel acted as working electrode. A graphite rod was used as counter electrode, and a silver chloride electrode (SCE) functioned as reference electrode. Linear potentiodynamic potential scan range was from -1.5V to +1.5 mV, at a scan rate of 0.0016 mV/s. The polarization potential (Ecorr), and current density (Icorr) data were evaluated from the Tafel plots. The surface coverage (θ) and the percentage inhibition efficiency (% IE) were calculated with equation 1 and 2 28.
The percentage inhibition efficiency (% IE) was calculated from corrosion current density values using equation 229,30.
where Icorr is the inhibited corrosion current densities and Iocorr is the uninhibited corrosion current density.
Results and discussion
Linear potentiodynamic polarization
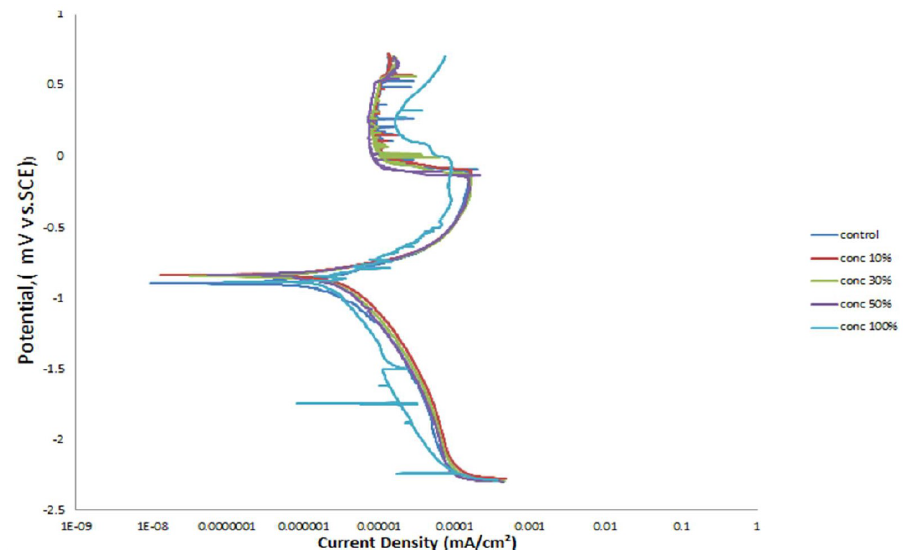
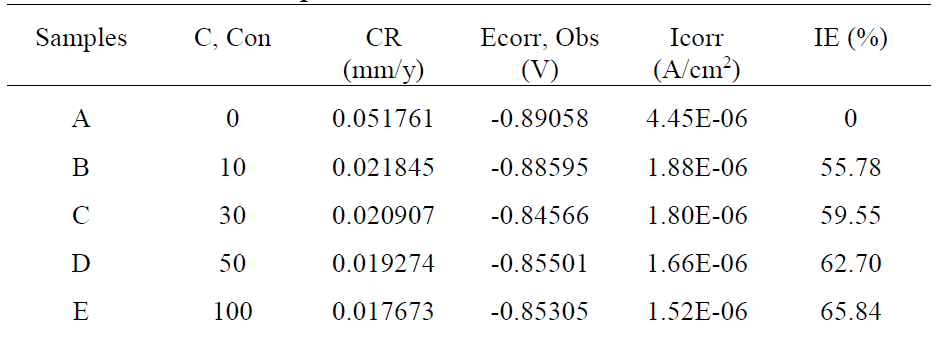
Mild steel potentiodynamic parameters in 3.65% NaCl, in the absence and presence of different inhibitor concentrations, are given in Table 2, and their corresponding polarization curves are shown in Fig. 2. This study revealed that the corrosion current density (Icorr) markedly decreased with the increase in the inhibitor concentration. However, the values of corrosion potential (Ecorr) shifted slightly to less negative values, upon addition of Cefalexin, indicating that it behaved as a mixed-type inhibitor, but predominantly as an anodic inhibitor in a NaCl solution, adsorbing its molecules onto the mild steel surface29,31.
Weight loss measurements
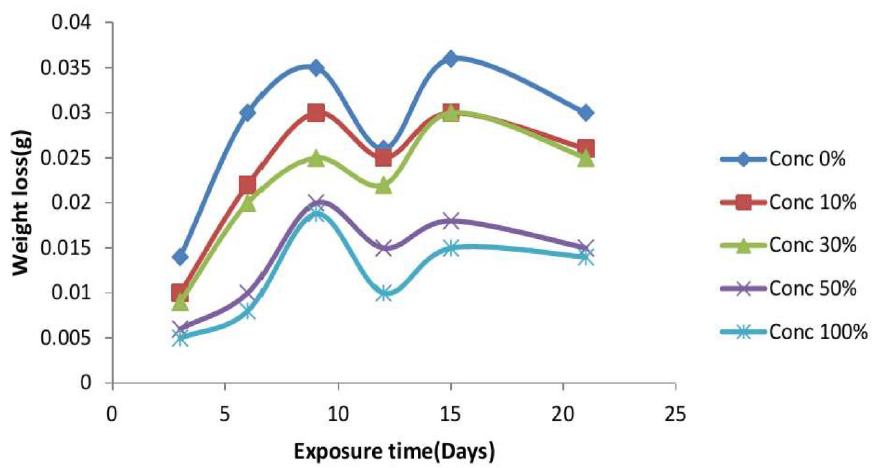
Results gotten from weight loss measurements are shown graphically in Fig. 3. The result indicates that the introduction of Cefalexin into the corrosive medium caused a significant reduction in mild steel corrosion and wear32. However, the weight loss of the inhibited and uninhibited samples increased with time, but it was minimal for the inhibited samples. The minimal weight loss is as a result of the ability of Cefalexin to form an adhesive synergy on the metal surface, like some other antibiotics33.
Mechanism of inhibition efficiency and adsorption study
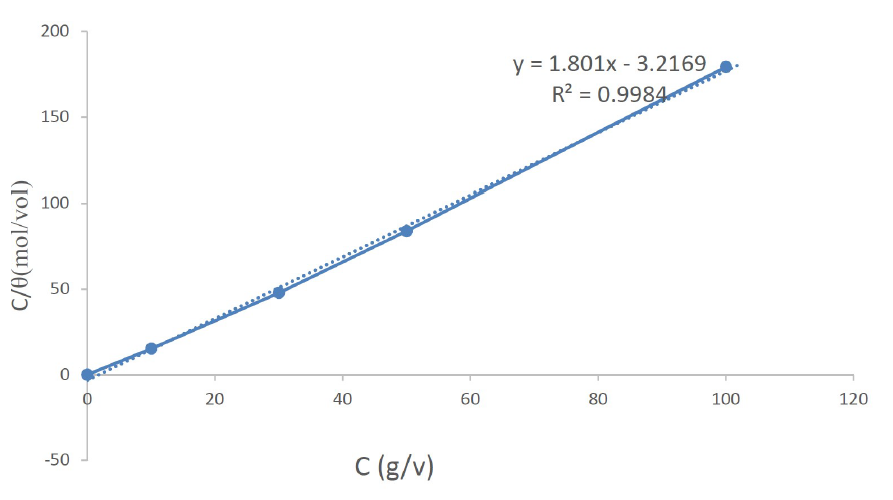
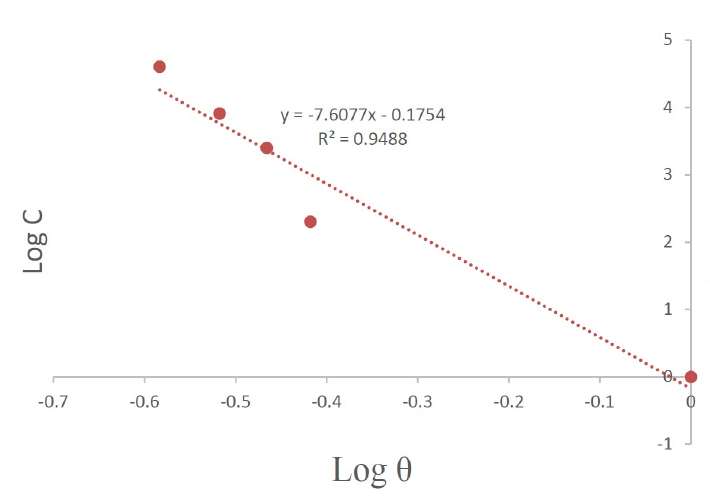
The values of corrosion current (Icorr) and corrosion rates (CR) were found to reduce with an increase in Cefalexin concentration, as shown in Table 2, indicating its adsorbent characteristic onto the mild steel surface34. The corrosion inhibition efficiency of Cefalexin increases with an increase in concentration. In order to have a good knowledge of the metal-inhibitor interaction and the metallic-complex activities on the coverage site, an adsorption mechanism facilitated the computation of C/θ and C for potentiodynamic polarization, using Langmuir isotherm and a linear relationship35,36. Equation (3) and (4) show the Langmuir and Freundlich isothermal, respectively. The Langmuir and Freundlich plots, in Fig. 4 and Fig. 5, on the surface features, show a linear relationship with the increase in the inhibitor concentration, indicating its continued adsorption onto the steel surface. The values of (R2) for Langmuir and Freundlich are 0.9984 and 0.9488, respectively, which are in accordance with the works of37,38. This shows that the mild steel corrosion protection by Cefalexin had been achieved, since R2 is close to unity.
The Langmuir isothermal law is38,39:
The Freundlich isotherm law is40:
where C is the concentration of the corrosion inhibitor, 𝜃 is the degree of surface coverage and 𝑘 is the adsorption equilibrium constant.
Conclusions
1. Langmuir isotherm and Freundlich isotherm law were obeyed by the inhibitor, with a correlation regression coefficient of R2= 0.9984 and R2= 0.9488. The values of R2 are near unity, indicating the effectiveness of the inhibitor.
2. Maximum corrosion inhibition efficiency of 65.84% was obtained for the inhibited mild steel in a salt solution. There is a tendency of further increment in inhibition efficiency, if the concentration of Cefalexin is increased.
3. The polarization studies showed that Cefalexin acted as a mixed-type inhibitor. This is an indication that the cathodic and anodic reactions were affected by the inhibitive drug. However, the anodic effect was predominant.
4. The corrosion of mild steel substrate in the chloride environment was mitigated by the effective adsorption of Cefalexin molecules.