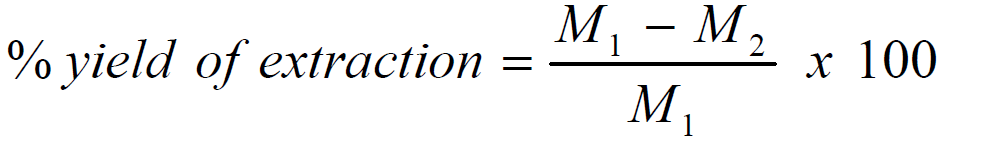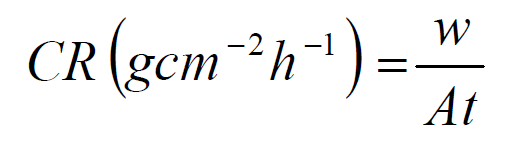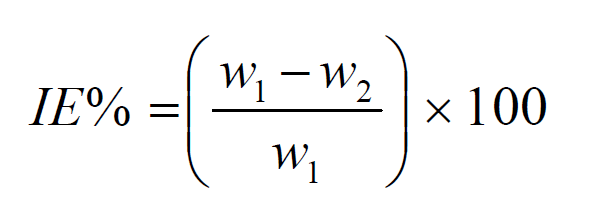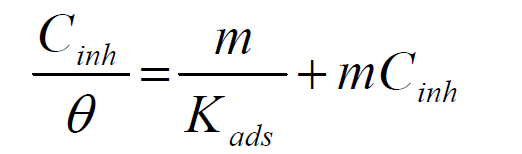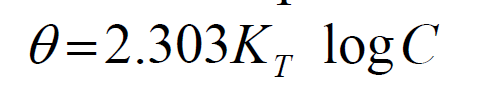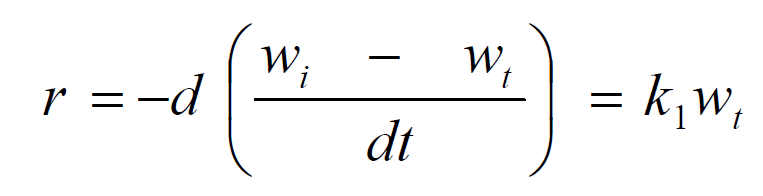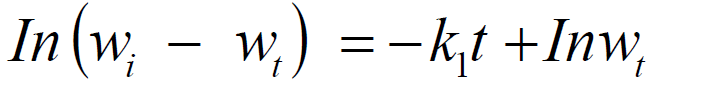Introduction
The corrosion process is a naturally occurring phenomenon which daily affects our society and results into damage, destruction and degradation of household gadgets, automobiles, airplanes, highway bridges, military armored and petrol tanks, among others. High-impurity products can be originated during the crude oil extraction, which might enhance corrosion. In the case of oil and gas wells and pipelines, corrosive products, such as carbon dioxide (CO2), hydrogen sulfide (H2S) and free water can cause corrosion to them [1]. CO2, H2S and free water continual extraction from oil and gas components can damage the inner surface of oil and gas wells and pipelines. This material degradation results in the loss of mechanical properties, such as strength, ductility, impact strength, and so on, which leads to materials damage, reduction in thickness and, at times, ultimate failure. If the degradation continues, the component may completely break down and require replacement. The serious consequences of the corrosion process became a problem worldwide [2].
Successful developments of researches to obtain natural corrosion inhibitors are growing simultaneously, as the demand for environmental protection has become higher [3]. Many noticeable research works have been published with the aim of developing non-hazardous corrosion inhibitors referred to as “green” corrosion inhibitors [4, 5]. Also, there has been an increasing research on green plants, such as plants extracts, essential oils and purified compounds, to obtain non-toxic and cheap corrosion inhibitors [6]. El-Etre [7] investigated Baphia nitida leaves extract as a good mild steel corrosion inhibitor in 1 M H2SO4, while Nnanna et al. [8] reported the effect ofEuphorbia hirta and Dialum guineenseleaves extracts on aluminium corrosion in 0.25 M NaOH. It has been shown that there is an inhibition effect of the Daucus carota aerial part extract on mild steel corrosion in a 1 M hydrochloric acidic solution [9].
The possible application of Pentaclethra macrophylla Bentham leaves extracts as a corrosion inhibitor, as well as the inhibition effect of the natural onions juice extract of khillah (Ammi visnaga) on zinc corrosion, have been investigated [10, 11]. The Punica granatum extract corrosion inhibition efficiency, on brass in an acidic media, and that of fennel Foeniculum vulgare essential oil, on carbon, have been investigated [12, 13]. The corrosion inhibition efficiency of green inhibitors on aluminium and alloys has been studied [14]. The corrosion inhibition properties of Raphia hookeri gum-halide mixtures on mild steel in an alkaline medium, using polyvinyl alcohol, have been studied [15].
However, there has been little study on the P. polyandra corrosion inhibition effect, with the exception of its leaves extract, on mild steel in a sulphuric acid solution, which is not detailed [16]. Therefore, this work emphasized the detailed investigation of P. polyandra leaves extract corrosion inhibition effect onto a mild steel surface in an acidic medium, using weight loss and electrochemical techniques, as well as kinectics, thermodynamics and adsorption isotherms studies.
Experimental
Corrosive solution and mild steel specimens preparation
The corrosive solution used in this study was a 1 M HCl solution prepared by measuring 86.0 mL (97 % purity of analytical grade) of the concentrated acid into a 1000 mL standard flask, and made up to the mark with the deionized water.
Mild steel sheets were purchased from Iron Sheet merchant, at Tanke Oke - Odo, Ilorin Kwara State, Nigeria. They were found to have the following composition (wt %): Fe (98.89 %), Ti (0.73%), Mn (0.20 %), Al (0.15%), P (0.011%), Mo (0.006%), W (0.0047%) and V (0.0024%), and they were cut into the same size (2.5 x 2.0 x 0.85 cm). The mild steel sheets were mechanically polished in separate, with Sic emery papers of grades 200, 400 and 600, in successive sections, washed with deionized water, degreased with ethanol, dried in acetone and kept in moisture free desiccators, prior to each carried out experiment [17].
Collected and extracted P. polyandra leaves, commonly known as abeere in yoruba, were obtained from the University of Ilorin, Ilorin, Nigeria, at a latitude of 8.4807o N and a longitude of 4.527o E. The leaves were identified at the Herbarium in the Department of Plant Biology, University of Ilorin, Nigeria. 70.0 g of P. polyandra leaves were washed, dried, grounded and soaked in a 500 mL solution of ethanol (97% purity), for 48 h. After 48 h, the samples were filtered, and the obtained filtrates were further subjected to evaporation at 352 K, in order to make them free of ethanol. The extract stock solutions were used to prepare different extract concentrations, by dissolving 0.1, 0.2, 0.3, 0.4 and 0.5 g of it in 1 L of 1 M HCl, for gravimetric and electrochemical analyses [18]. The leaves extraction percentage yield was calculated by using the formula [19]:
where M1 is the sample and the thimble mass before extraction and M2 is the sample and the thimble mass after extraction.
P. polyandra leaves extracts physicochemical and spectroscopic properties determination
Physicochemical parameters, such as yield (%), pH, conductivity (µS/cm), ash content (%), moisture content (%) and bulk density (g/mL), were determined using standard methods [5]. The P. polyandra leaves extracts Fourier Transform spectroscopic analysis was also done.
Scanning Electron Microscopy (SEM) studies
Two cleaned mild steel specimens were immersed separately in 50 mL of the blank (uninhibited 1 M HCl) and inhibited 1 M HCl solutions, with 0.2 g/L of the extract, for 48 hours, at room temperature (303 K), after which they were removed and dried in air. The dried mild steel sheets were then subjected to scanning electron microscopy (SEM), using a high vacuum Based SEM SCOTECH 30000 SEM (Germany), for obtaining the visual physical surface morphology of the uninhibited sheets and of those inhibited with P. polyandra leaves extract [10].
Corrosion inhibition effects measurement using weight loss studies
Each pre-weighed mild steel sheet was separately immersed in 50 mL of 1 M HCl without inhibitor (blank solution) and with different inhibitor leaves extract concentrations (0.1, 0.2, 0.3, 0.4, and 0.5 g/L). The beakers were placed in a thermostated water bath [5]. The experiments were conducted at various temperatures of 303, 313, 323, 333 and 343 K, for 10 h each.
Corrosion rate (CR) and percentage corrosion inhibition efficiency were calculated using the equations 2 and 3:
where w, A and t are weight loss (mg), exposed area (cm2) and minimum time (h), respectively, while w1 and w2 indicate the mild steel original weight and weight loss in either an uninhibited solution (blank) or an inhibited solution with the P. polyandra leaves extract.
Corrosion effects measurement using electrochemical studies
The electrochemical studies were performed using a VERSASTAT 400 complete dc voltammetry and corrosion system model with V3 Studio software. The mild steel was cut into a 1 cm2 square area which was exposed to the corrosive media, with and without inhibitors, as working electrode, and an Ag/AgCl rod as counter electrode. A saturated calomel electrode (SCE) was used as reference electrode, and it was connected by a Luggin’s capillary. The experiments were undertaken at room temperature (303 K). The working electrode was immersed in a test solution for 1 h, until a stable open circuit potential was attained. The Tafel analysis study was set from a cathodic potential of -250 mV to an anodic potential of +250 mV, with respect to the corrosion potential, at a sweep rate of 1 mV/s. The linear Tafel segments of the anodic and cathodic curves were extrapolated to corrosion potential, to obtain the corrosion current densities (icorr). Each experiment was carried out three times to estimate the electrochemical parameters reproducibility and average values which are reported [20].
Results and discussion
The yield is regarded as the quantity of product formed per the quantity of starting materials. Table 1 shows that P. polyandra leaves extract yield was 19.82 %, and its pH 4.8.
Table 1 P. Polyandra leaves (raw and extracts) physicochemical properties.
| Physicochemical properties | Raw leaves | Leaves extract | ||
|---|---|---|---|---|
| Yield (%) | * | 19.82 | ||
| pH | 5.3 | 4.8 | ||
| Conductivity (µS/cm) | 714.19 | 431.24 | ||
| Ash content (%) | 6.34 | 7.21 | ||
| Moisture content (%) | 14.53 | 2.45 | ||
| Bulk density (g/ml) | 0.32 | 0.41 |
* not applicable; percentage error for each parameter is less than 5%.
The P. polyandra leaves extract inorganic mineral content, which represents the ash percentage, was found to be 7.21%, while its moisture content was 12.45%. The obtained extract conductivity was 431.24 µS/cm, and its bulk density was 0.41g/mL. It could also be observed that the raw leaves pH is comparable to that of their corresponding extract, as both fell within an acidic region of 5.3 and 4.8 pH, respectively. All raw leaves various parameters values were found to be higher than those of their corresponding extracts, with the exception of the ash content and bulk density values that were found to be greater in the leaves extracts. The increase in the leaves extracts ash content might be due to the chemical reagents used during their treatment.
FTIR analysis
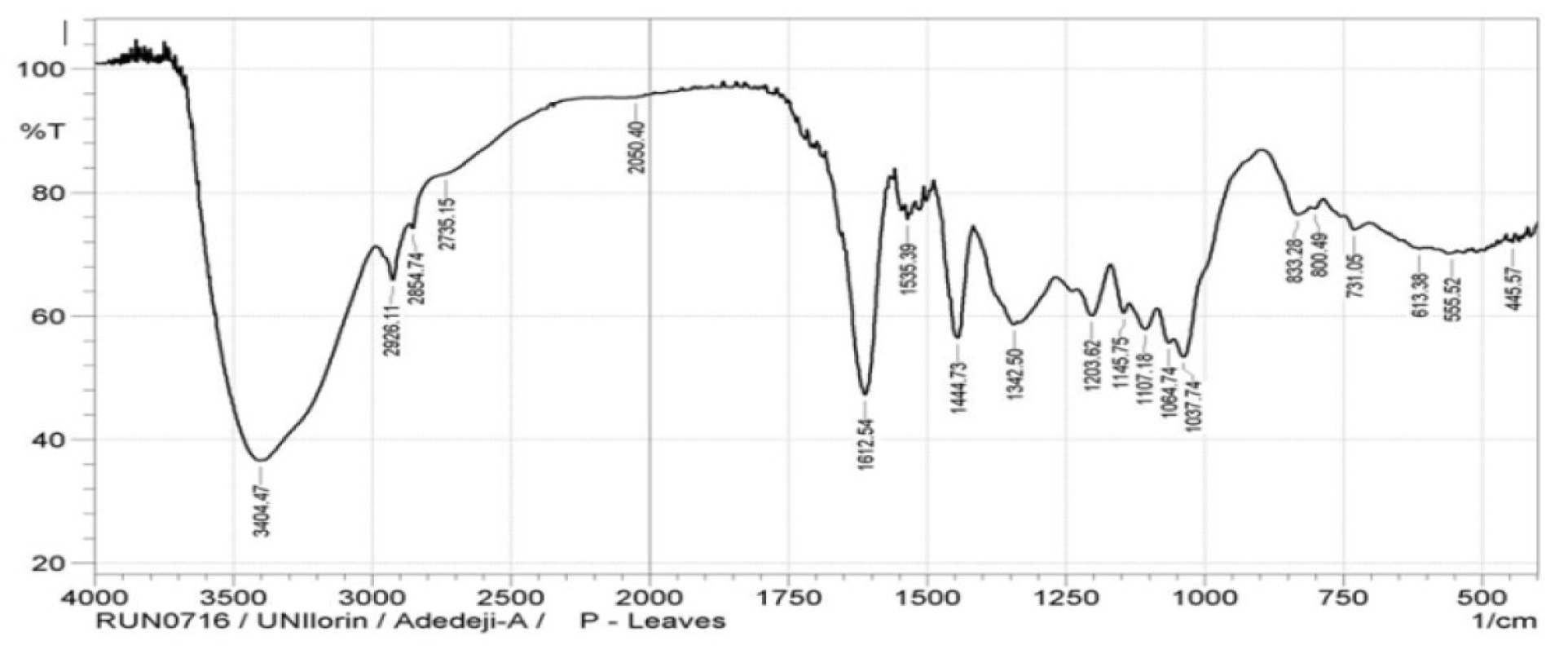
The P. polyandra leaves extracts micrograph of the FTIR analysis is shown in Fig. 1, while the assignment of important bands of this inhibitor is presented in Table 2.
Table 2 Assignment of the important peaks observed in the P polyandra leaves extracts.
| Absorption band location of P. polyandra leaves (cm-1) | Types of vibration and assignments |
|---|---|
| 3404 | OH stretching vibration of phenolic group |
| 2926 | C-H stretching vibration of methoxyl group |
| 1612 | C=C ring stretch (conjugated) in aromatic |
| 1444 | O-H bend of carboxylic acids |
| 1342 | C-C stretch in ketones |
| 1203 | C-C(O)-C stretch of esters |
A strong band at 3404 cm-1 was discovered and assigned to phenolic OH stretching vibration of the phenolic groups involved in the hydrogen bond. The bands at 2924 cm-1 were assigned to C-H stretching vibrations of the methoxyl group in P. polyandra leaves extract.
At the band of 1444 cm-1, OH bend in carboxylic acid was observed in P. polyandra leaves extract, while the band at 1342 cm-1 revealed the C-C stretch of ketones in the inhibitor. Bands at 1203 cm-1 and 1220 cm-1 were assigned to the C-C (O)-C stretching vibrations of esters in bonds in aliphatic ethers of P. polyandra leaves extracts. The absorption bands at 833 cm-1 - 555 cm-1 indicate the hetero-atoms presence in the inhibitor.
Corrosion studies
The corrosion studies involve the assessment of a given metal material loss extent, when it is exposed to certain harsh conditions. Although the gravimetric method is not very reliable, it is time consuming and cannot effectively predict the corrosion reaction mechanism, corrosion measurements can also be carried out using electrochemical techniques [19]. Table 3 indicates the various data obtained from the gravimetric method of mild steel weight loss calculations, in 1 M HCl of uninhibited and inhibited solutions, with different inhibitors concentrations, at various temperatures.
Table 3 Mild steel corrosion parameters in 1 M HCl, with various leaves extract concentrations, at different temperatures, using weight loss technique.
| LEC (g/l) | W L (g) | Temperature (K) | C R (gcm-2 h-1) | Ѳ | IE (%) |
|---|---|---|---|---|---|
| Uninhibited | 1.1011 | 303 K | 0.0404 | N.A | N.A |
| 0.1 | 0.4875 | 0.0195 | 0.09475 | 94.75 | |
| 0.2 | 0.2646 | 0.0195 | 0.9722 | 97.22 | |
| 0.3 | 0.4653 | 0.0186 | 0.9529 | 95.29 | |
| 0.4 | 0.5788 | 0.0231 | 0.9404 | 94.06 | |
| 0.5 | 0.4553 | 0.0182 | 0.9539 | 95.39 | |
| Uninhibited | 1.4399 | 313 K | 0.0575 | N.A | N.A |
| 0.1 | 0.9562 | 0.0382 | 0.8991 | 89.91 | |
| 0.2 | 0.8546 | 0.0341 | 0.9192 | 91.92 | |
| 0.3 | 0. 7825 | 0.0313 | 0.9197 | 91.97 | |
| 0.4 | 0.6439 | 0.0257 | 0.9317 | 93.17 | |
| 0.5 | 0.5112 | 0.0204 | 0.9491 | 94.91 | |
| Uninhibited | 1.6502 | 323 K | 0.0600 | N. A | N. A |
| 0.1 | 1.1232 | 0.0449 | 0.8871 | 88.71 | |
| 0.2 | 1.0056 | 0.0402 | 0.8929 | 89.29 | |
| 0.3 | 0.8481 | 0.0339 | 0.9104 | 91.04 | |
| 0.4 | 0.7931 | 0.0317 | 0.9230 | 92.30 | |
| 0.5 | 0.6215 | 0.0248 | 0.9430 | 94.30 | |
| Uninhibited | 2.3215 | 333 K | 0.0986 | N. A | N. A |
| 0.1 | 1.4312 | 0.0502 | 0.8585 | 85.85 | |
| 0.2 | 1.2556 | 0.0502 | 0.8871 | 88.71 | |
| 0.3 | 1.0563 | 0.0422 | 0.8920 | 89.20 | |
| 0.4 | 0.9825 | 0.0393 | 0.9059 | 90.59 | |
| 0.5 | 0.7784 | 0.0311 | 0.9219 | 92.19 | |
| Uninhibited | 3.1358 | 343 K | 0.1254 | N. A | N.A |
| 0.1 | 1.7842 | 0.0713 | 0.8203 | 82.03 | |
| 0.2 | 1.6045 | 0.0642 | 0.8339 | 83.39 | |
| 0.3 | 1.5213 | 0.0608 | 0.8404 | 84.04 | |
| 0.4 | 1.3786 | 0.0551 | 0.8514 | 85.14 | |
| 0.5 | 1.2015 | 0.0480 | 0.8780 | 87.80 |
LEC = leaves extracts concentration, WL = weight loss, CR = corrosion rate, θ = degree of surface coverage, IE = inhibition efficiency, NA = not applicable.
The corrosion rate was found to decrease with higher inhibitor concentrations and temperatures. The temperature of 303 K and the increase in P. Polyandra leaves extract concentrations, from 0.1 g/L to 0.5 g/L, caused the mild steel corrosion rate to decrease from 0.0195 to 0.0182 gcm-2 h-1 (as seen in its plots of Fig. 2), in line with the results obtained in literature [20].
Akpan and Offiong [21] also reported a similar trend of the results, when ciprofloxacin drug was used as an eco-friendly inhibitor.
Also, the P. Polyandra leaves extracts inhibition efficiency (IE) ranged from 97.22 to 82.03% (Table 3). The EI increased gradually with higher inhibitor concentrations, but decreased with a rise in temperature. Maximum inhibition efficiency of 97.22 % was obtained at 0.2 g/L of P. Polyandra leaves extracts in a 1 M HCl solution, at a temperature of 303 K.
A similar trend has been reported in literature [22], when red onion skin acetone extract was used as a corrosion inhibitor for zinc in a 2 M hydrochloric acidic solution.
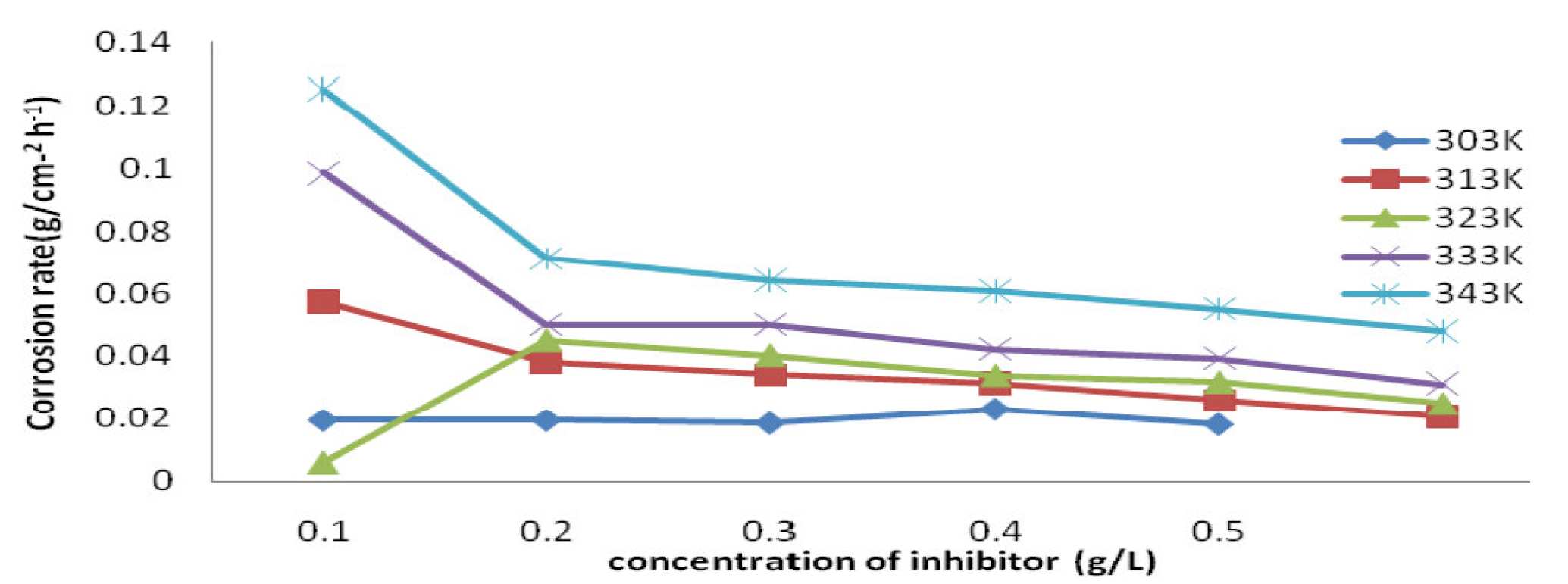
Figure 2 P. polyandra leaves extracts concentration effect on mild steel corrosion rate, in 1 M HCl, at various temperatures (303 - 343 K).
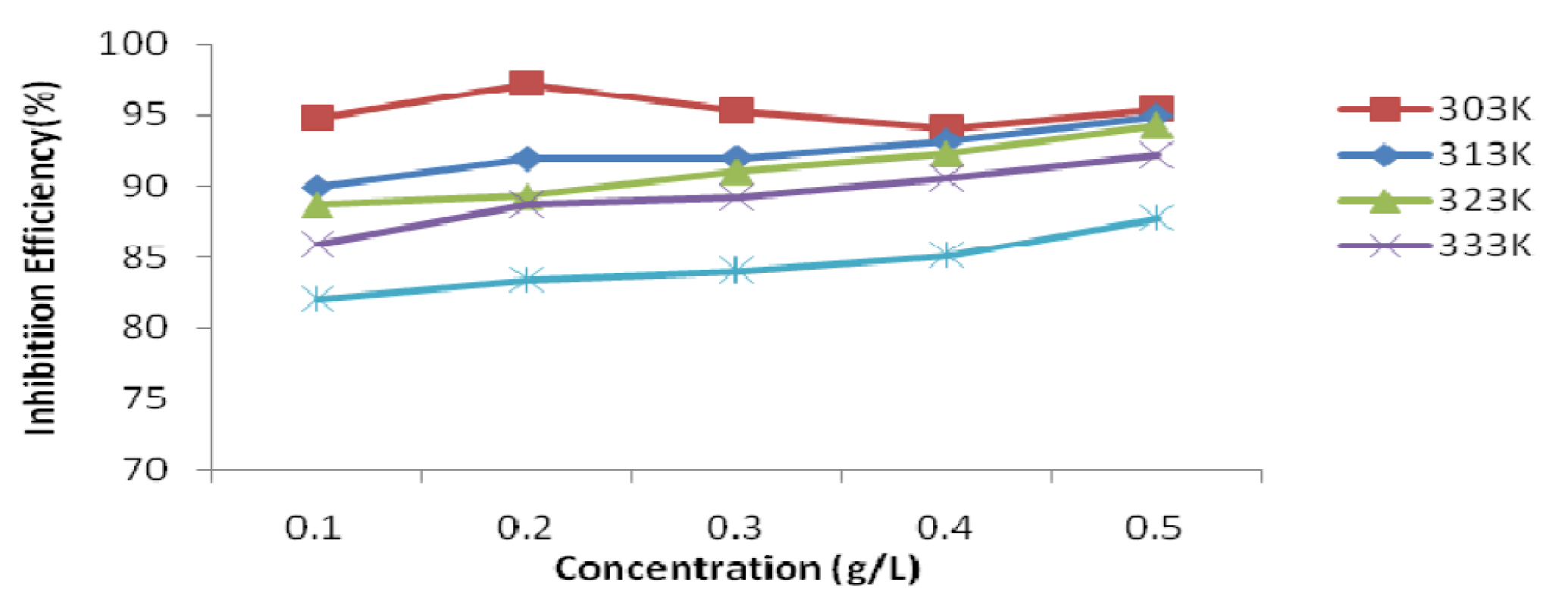
Figure 3 P. Polyandra leaves extract concentration effect on its inhibition efficiency on mild steel corrosion in 1 M HCl, at a temperature range from 303 to 343 K.
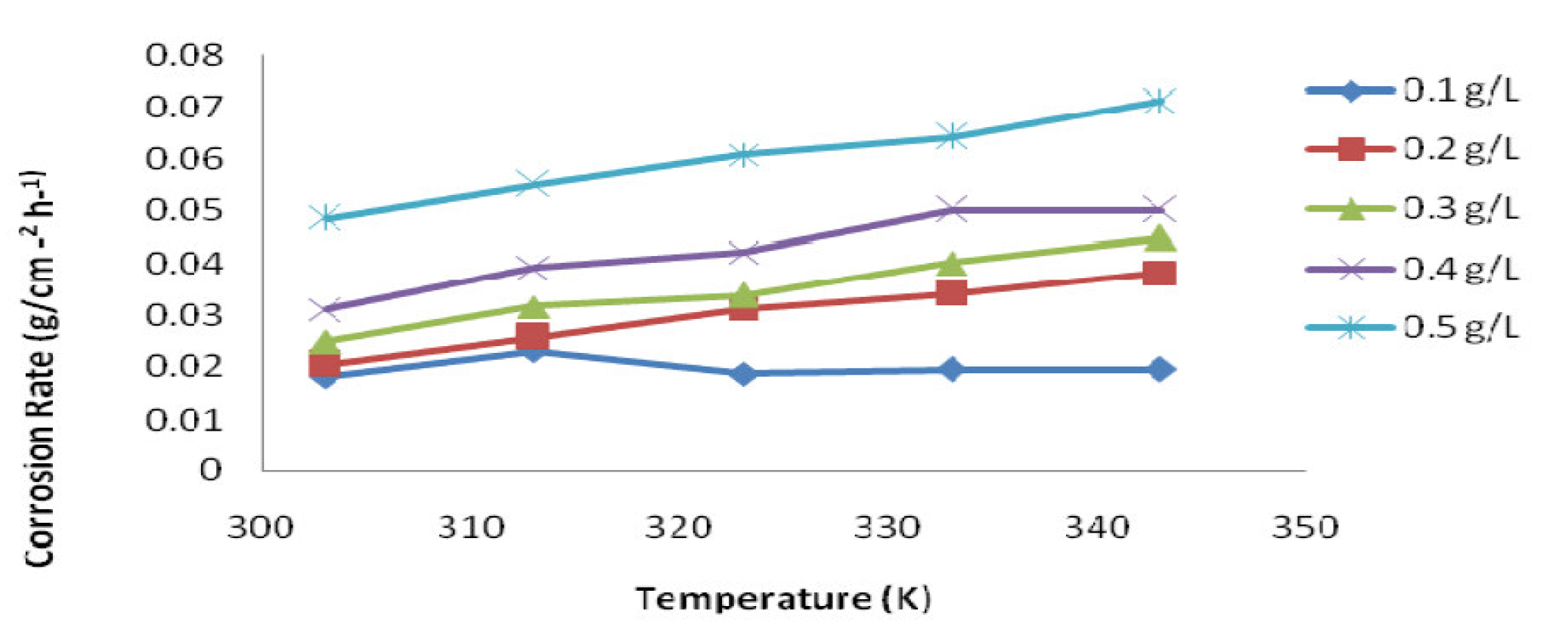
Figure 4 Temperature effect on mild steel corrosion rate in 1 M HCl inhibited with Parinari Polyandra leaves extract, at various temperatures (303 - 343 K).
The IE variation with P. polyandra leaves extract concentrations at various temperatures, ranging from 303 K to 333 K, is shown in Fig. 3. It can be observed that, as temperature increased, the P. polyandra leaves extract inhibition effect on the mild steel corrosion decreased, with the various investigated concentrations (Fig. 3). This is because the tendency of the leaves extract molecules to be attached to the mild steel surface decreases with higher temperatures [22].
Fig. 4 depicts the corrosion rate of mild steel inhibited with various P. polyandra leaves extract concentrations, at different temperatures.
Immersion time effect
The immersion time effect on mild steel corrosion rate (CR) and on inhibition efficiency (IE) in a 1 M HCl corrosive environment is shown in Table 4. The CR, in uninhibited and inhibited HCl, and IE, in the presence of leaves extracts maximum concentration, at room temperature, were calculated using their respective formulae, at a 3 days interval, for a total of 18 days. Mild steel corrosion parameters, in 1 M HCl, without inhibitor and with 0.5 g/L of P. Polyandra leaves extracts, at different immersion times, at a temperature of 303 K, using weight loss techniques, are presented in Table 4.
Table 4 Time of immersion effect on the mild steel corrosion rate in uninhibited and inhibited 1 M HCl solutions, with various P. polyandra leaves extract concentrations.
| Inhibitor type | Immersion time (days) | W L (g) | W L (g) | CR (g/cm2h) | CR (g/cm) | θ | IE (%) |
|---|---|---|---|---|---|---|---|
| Uninhib | Inhib | Uninhib | Inhib | ||||
| Leaves extract | 3 | 0.132 | 0.103 | 0.052 | 0.020 | 0.9541 | 95.41 |
| 6 | 0.190 | 0.168 | 0.076 | 0.045 | 0.9022 | 90.22 | |
| 9 | 0.256 | 0.205 | 0.145 | 0.057 | 0.8615 | 86.15 | |
| 12 | 0.297 | 0.278 | 0.295 | 0.075 | 0.8392 | 83.92 | |
| 15 | 0.300 | 0.295 | 0.304 | 0.109 | 0.8040 | 80.40 | |
| 18 | 0.350 | 0.325 | 0.584 | 0.250 | 0.7521 | 75.21 |
WL means weight loss, CR is the corrosion rate, θ represents surface coverage and IE is inhibition efficiency. Temperature is at 303 K.
From Table 4, it is observed that MS corrosion rate in uninhibited 1 M HCl increased from 0.052 g.cm-2h-1, after 3 days of immersion, to 0.584 g.cm-2h-1, after 18 days of immersion.
This trend also occurred when a 1 M HCl solution was inhibited with the P. Polyandra leaves extract, as the corrosion rate went from 0.020 g.cm-2h-1, after 3 days of immersion time, to 0.250 g.cm-2h-1, after 18 days of immersion time.
Fig. 5 shows the immersion time effect on the corrosion rate of mild steel immersed either in an uninhibited 1 M HCl solution or in 1 M HCl inhibited with 0.5 g /L P. polyandra leaves extract; at this concentration, the metal corrosion rate was the lowest (Fig. 5).
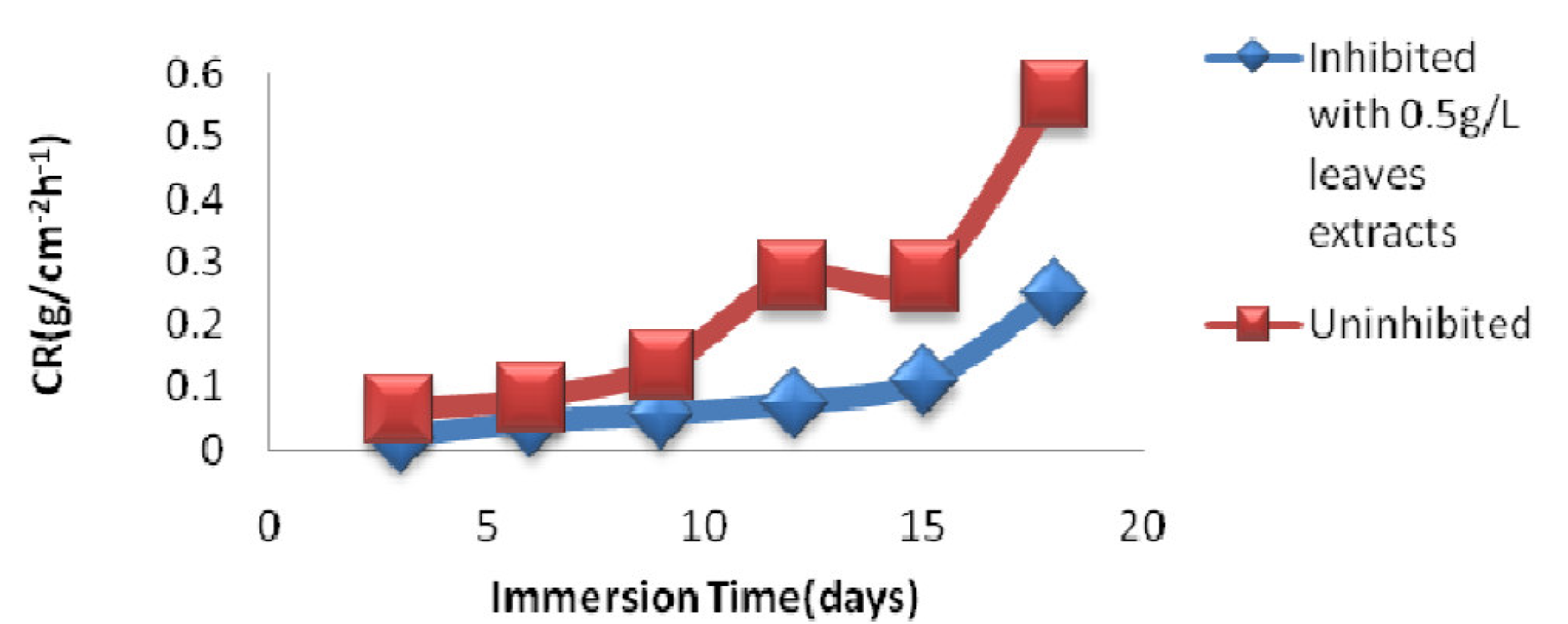
Figure 5 Time of immersion effect on the mild steel corrosion rate in an uninhibited 1 M HCl solution and in 1 M HCl inhibited with the 0.5 g/L leaves extracts solution.
Adsorption studies
Adsorption isotherms are very important in determining the mechanism of organo-electrochemical reaction. The corrosion inhibition of mild steel in a 1 M HCl medium with different P. polyandra leaves extract concentrations can be explained by the inhibitor components adsorption onto the metal surface. Inhibition efficiency (%) is directly proportional to the surface fraction covered by the adsorbed molecule (θ). Therefore, the adsorption isotherm describes the relationship between the interface coverage with the adsorbed species and the latter concentration in the solution. The extract surface coverage (θ) value at different inhibitors concentrations in a 1 M HCl solution was made to fit various adsorption isotherms.
Modified Langmuir adsorption isotherm for corrosion studies
Using this modified Langmuir expression shown in equation 4 [23]:
Langmuir plot of 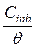 against Cinh gave straight lines for the 1 M HCl solution inhibited with P. polyandra leaves extracts. Figs. 6 and 7 clearly show that the leaves extract adsorption onto the mild steel surface fitted very well the modified Langmuir equation, as the inhibitor correlation coefficients fell within the range from 0.998 to 0.999, at the temperature range from 303 to 343 K. Table 5 summarizes the obtained Langmuir parameters.
against Cinh gave straight lines for the 1 M HCl solution inhibited with P. polyandra leaves extracts. Figs. 6 and 7 clearly show that the leaves extract adsorption onto the mild steel surface fitted very well the modified Langmuir equation, as the inhibitor correlation coefficients fell within the range from 0.998 to 0.999, at the temperature range from 303 to 343 K. Table 5 summarizes the obtained Langmuir parameters.
It is known that KLads describes the inhibitor adsorption strength onto the mild steel surface [5]. The large KLads values suggest an efficient inhibitor binding with the mild steel surface [24]. In this study, the KL value could be seen to slightly increase as the leaves extracts temperature rose from 303 to 343 K. The lowest KLads value obtained for the leaves extracts, at 303 K, was found to be 1.0499 g/L.
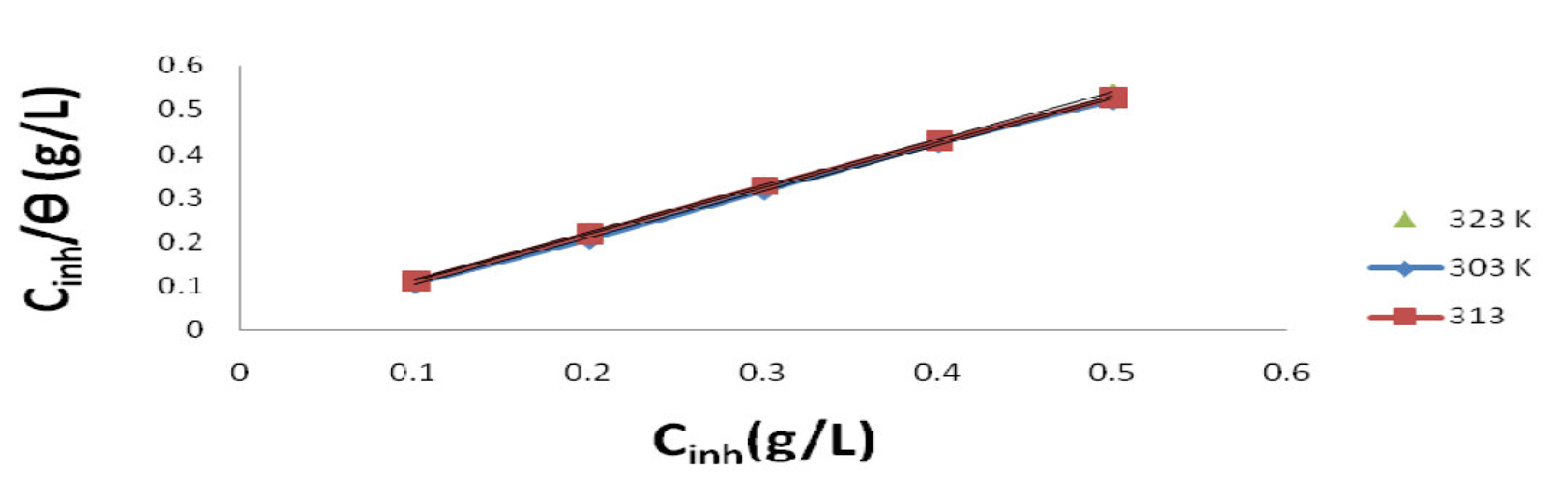
Figure 6 Langmuir plot for mild steel in a 1 M HCl solution inhibited with different P. Polyandra leaves extract concentrations, at various temperatures.
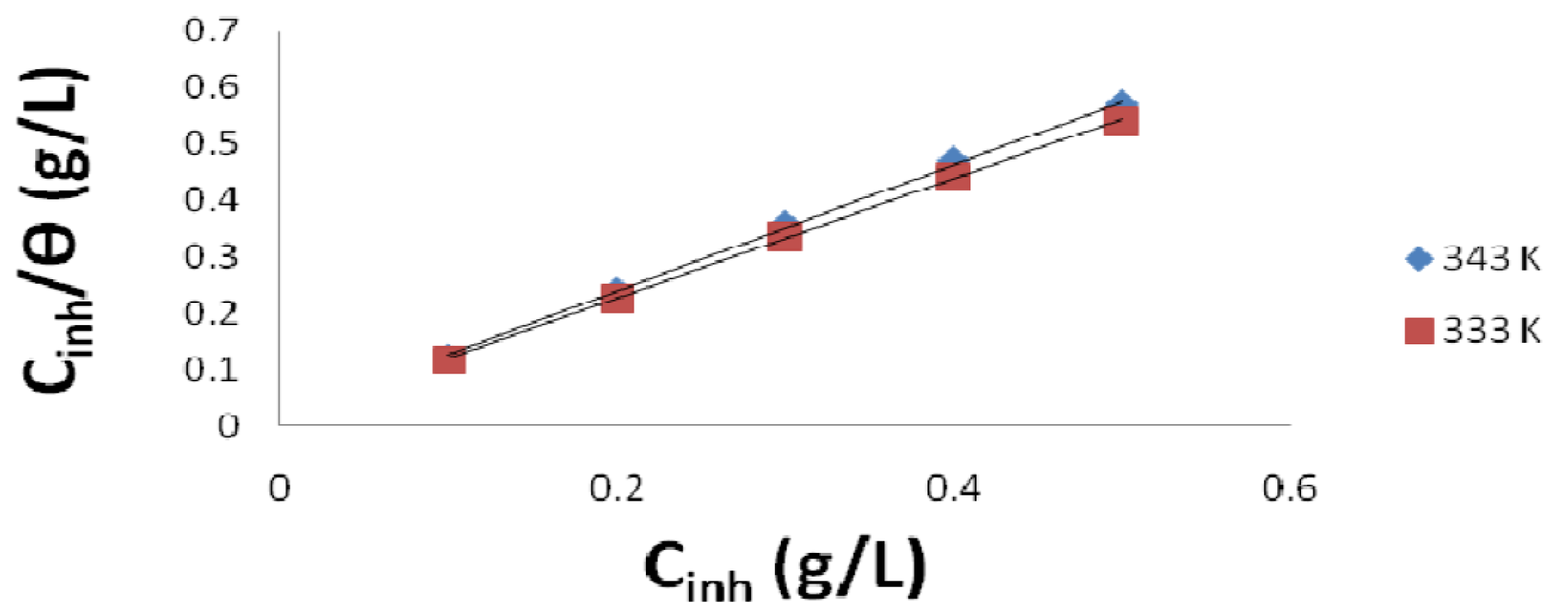
Figure 7 Langmuir plot for mild steel in a 1 M HCl solution inhibited with different P. Polyandra leaves extract concentrations, at two temperatures.
Table 5 Langmuir parameters for mild steel in a 1 M HCl solution inhibited with different P. polyandra leaves extract concentrations, at various temperatures.
| Leaves extract | ||||||
|---|---|---|---|---|---|---|
| Temperature (K) | KLads (g/L) | m | R2 | |||
| 303 | 1.0499 | 1.051 | 0.999 | |||
| 313 | 1.0509 | 1.043 | 0.999 | |||
| 323 | 1.0669 | 1.059 | 0.999 | |||
| 333 | 1.0779 | 1.067 | 0.999 | |||
| 343 | 1.1350 | 1.124 | 0.998 | |||
KLads = Langmuir adsorption constant, m= a numerical value representing the slope.
Temkin adsorption
Temkin adsorption isotherm, as applied to corrosion studies, takes into account the interaction between adsorbent and adsorbate ions or molecules, and it is based on the assumption that the free energy of adsorption is a function of surface coverage [12]. The equation of Temkin adsorption isotherm is given as:
where ( is the surface coverage, KT is the Temkin adsorption constant and C is the inhibitor concentration. The Temkin plot for the inhibitor adsorption onto the mild steel surface, in a 1 M HCl solution, at various temperatures, was done by plotting the surface coverage, θ, against Log C (Fig. 8). Temkin parameters are shown in Table 6.
The ( Temkin plots versus log Cinh, at various studied temperatures, of the leaves extract solution, are depicted in Fig. 8. The graphic is linear and KTads values increased at higher temperatures, while their correlation coefficient, R2, is in a good range from 0.962 to 0.825, with the exception of R2 = 0.024 (Table 6). A similar finding has been reported in literature [23, 24].
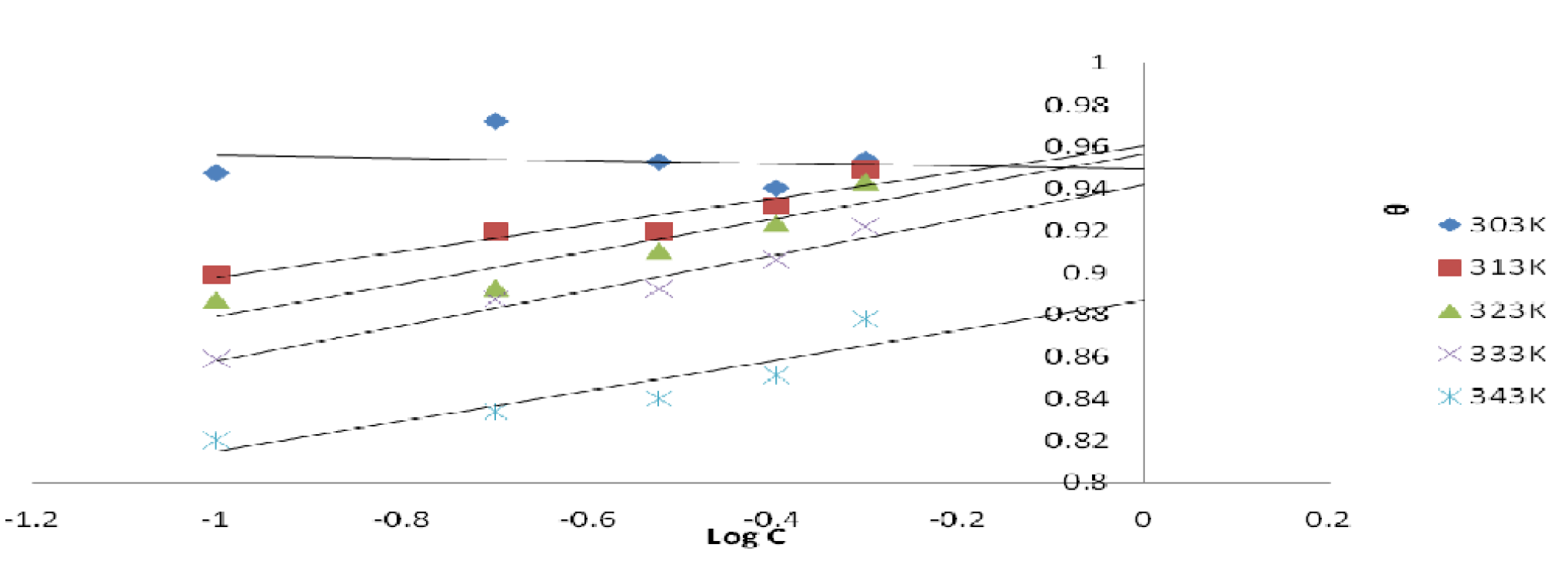
Figure 8 Temkin plot for mild steel in a 1 M HCl solution with different P. polyandra leaves extract concentrations, at various temperatures.
Table 6 Temkin parameters for mild steel in a 1 M HCl solution with different P. polyandra leaves extract concentrations, at various temperatures.
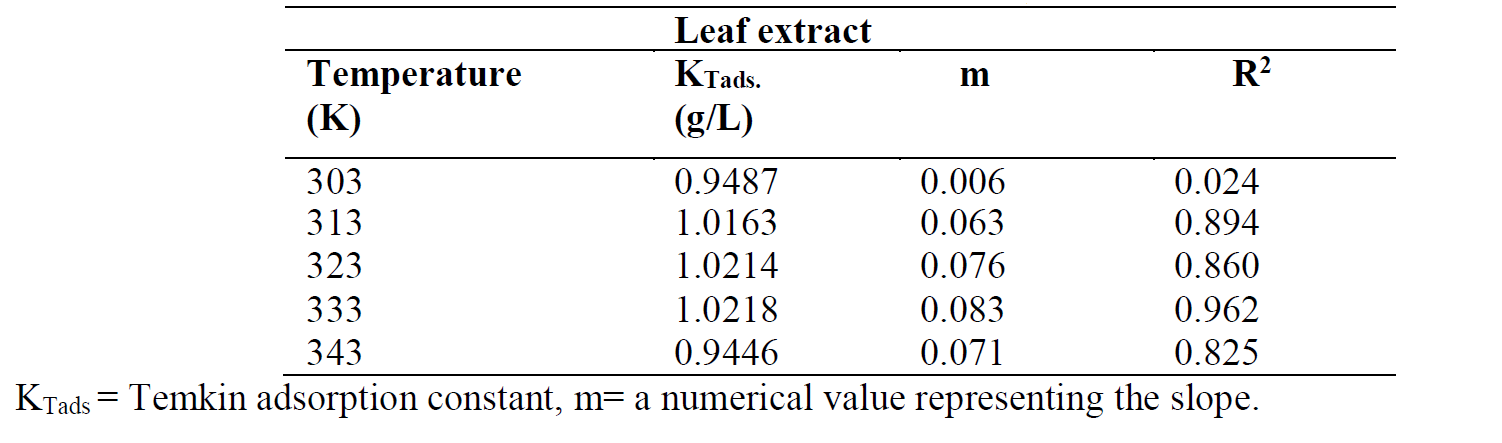
Among the three investigated adsorption isotherms, Langmuir was found to be the best to fit the corrosion adsorption data, by the virtue of its higher correlation coefficient, R2, value.
Thermodynamic studies
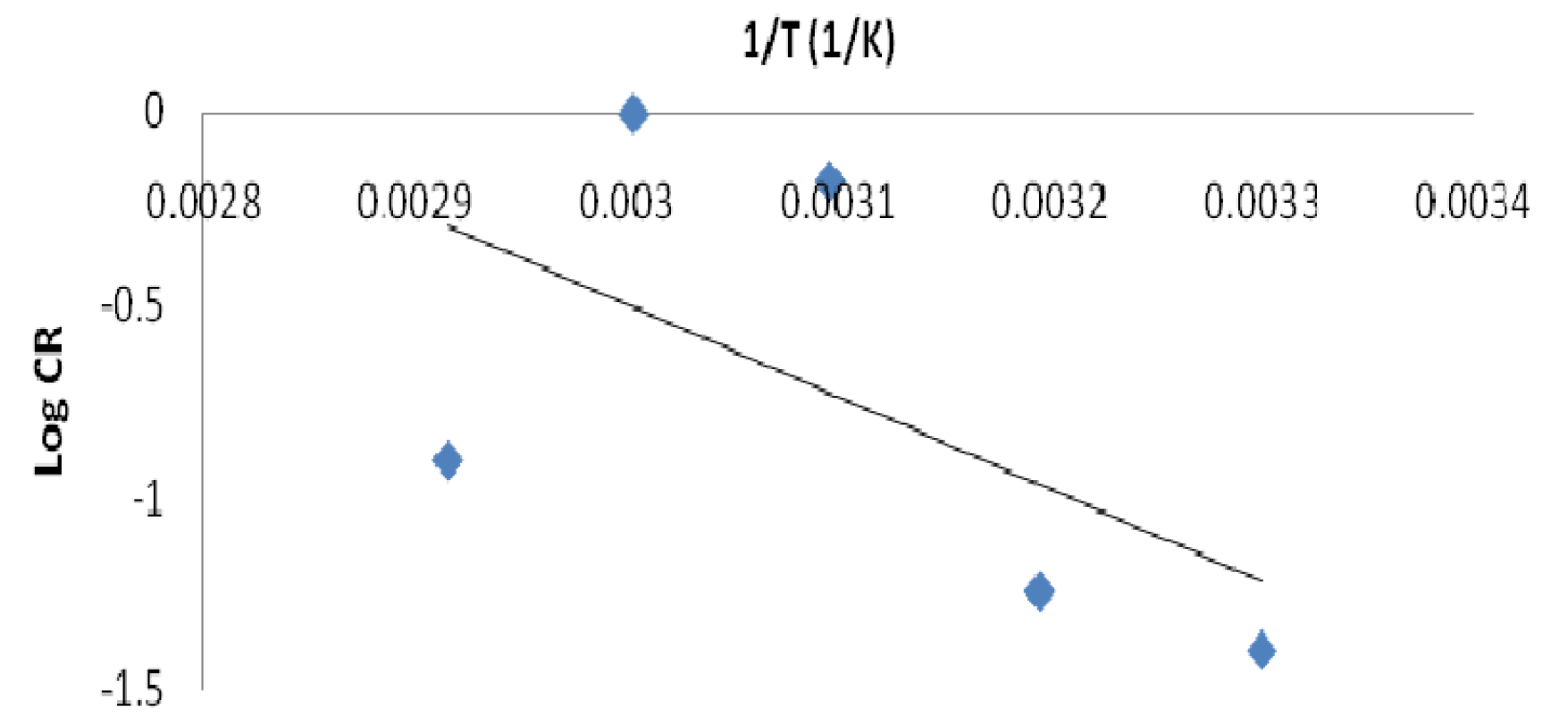
Thermodynamic properties, such as activation energy (Ea), enthalpy (∆H) and entropy of activation (∆S), show the involved adsorption mechanism. The Ea, ∆H and ∆S values obtained from the Arrhenius plot of Log CR against 1/T values are given in Tables 7 and 8. The activation energy (Ea) values decreased as the concentrations increased. The Ea of an uninhibited solution was found to be 45.79 kJ/mol. Raising the inhibitor concentration from 0.1 g/L to 0.4 g/L led to the Ea value reduction from 45.79 kJ/mol to 8.97 kJ/mol, at a concentration of 0.4 g/L. The addition of a higher leaves extract (inhibitor) concentration produced a better and thicker protective film layer on the mild steel surface, at a particular temperature.
Table 7 Activation parameters of mild steel dissolution in uninhibited 1 M HCl and in the presence of different leaves extracts concentrations.
| LEC (g/L) | Ea (kj / mol) | R2 | ∆H (kj / mol) | ∆S (j/mol K) | R2 |
|---|---|---|---|---|---|
| Uninhibited | 45.79 | 0.341 | 70.61 | -197.83 | 0.2169 |
| 0.1 g/L | 24.92 | 0.9126 | 104.69 | -146.95 | 0.9400 |
| 0.2 g/L | 24.05 | 0.9565 | 108.59 | -150.8 | 0.9300 |
| 0.3 g/L | 23.08 | 0.9481 | 66.83 | -153.90 | 0.7813 |
| 0.4 g/L | 8.97 | 0.6335 | 57.69 | -196.00 | 0.8841 |
| 0.5 g/L | 24.96 | 0.8398 | 105.8 | -199.00 | 0.9342 |
LEC = Leaves extract concentration, Ea = activation energy, ∆H = enthalpy of activation, ∆S= entropy of activation.
The trend of increase in the Ea value, at higher inhibitors concentrations, has been reported on various green plant extracts [19, 25].
The Ea values were used in this study to know the adsorption mechanism that took place [26]. It was found that the Ea constant or lower values (8.97 - 24.96 KJ/mol) in an inhibited system, compared to those of the blank solution (uninhibited), indicate an inhibition chemisorption process. So, an optimum lowered Ea value of the inhibited solution indicates the P. polyandra leaves extract inhibition efficiency, which is comparable to the values obtained in literature [5]. Therefore, the adsorption mechanism might be suggested to be chemisorption. The plot of log CR against the uninhibited solution temperature inverse produced a scattered graph with a very low correlation coefficient, R2 (Fig. 9).
Figs. 10a and b show the Arrhenius plot for mild steel in a 1 M HCl inhibited solution with different extract concentrations.
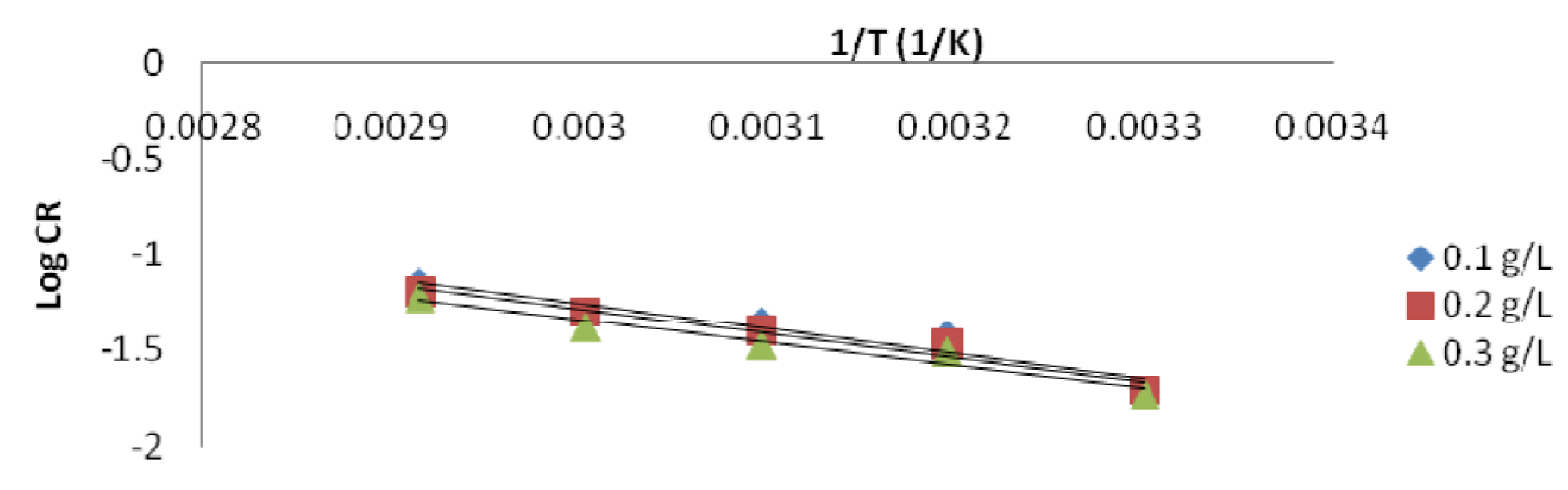
Figure 10a Arrhenius plot for mild steel in a 1 M HCl solution containing 0.1, 0.2 and 0.3 g/L P. Polyandra leaves extracts concentrations.
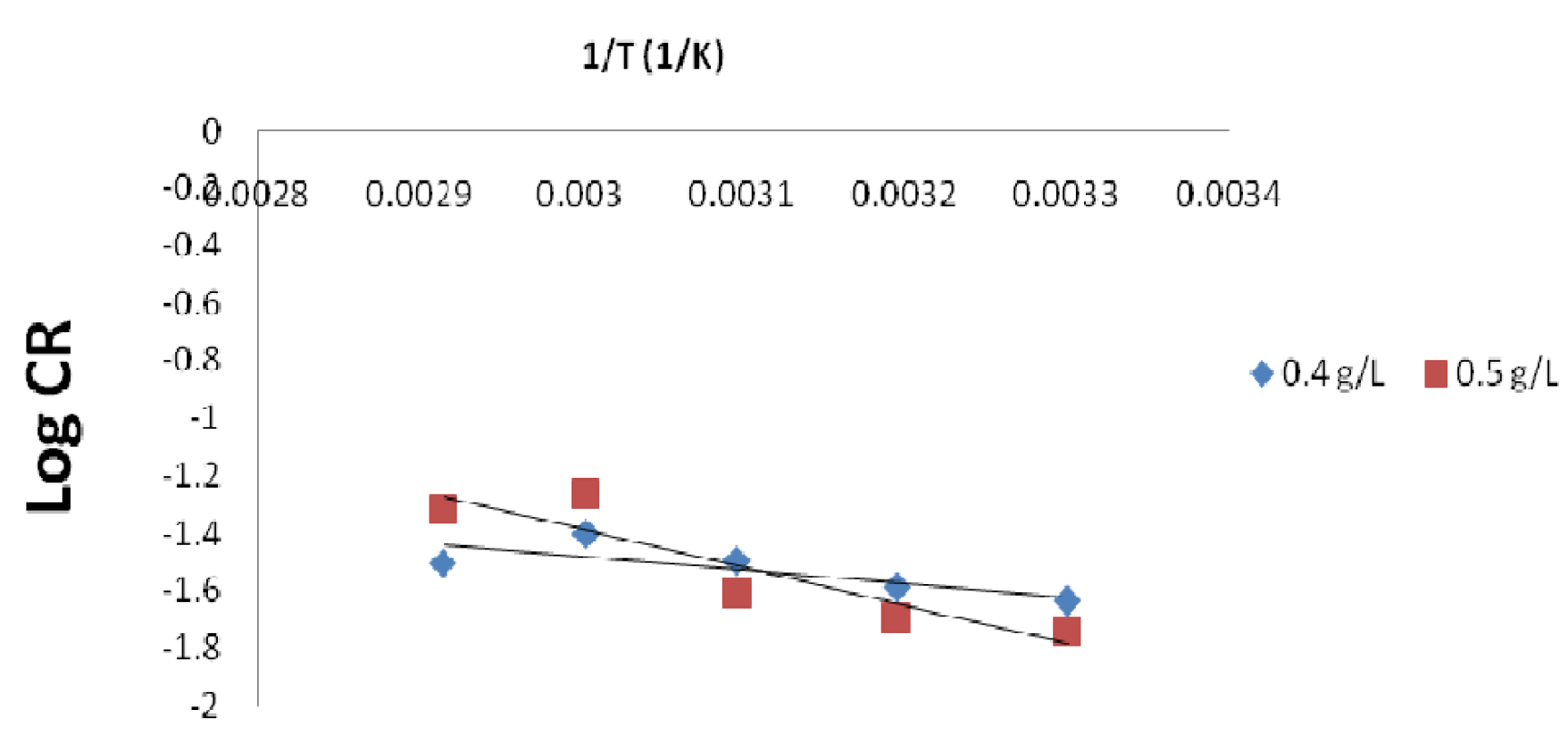
Figure 10b Arrhenius plot for mild steel in a 1 M HCl solution containing 0.4 and 0.5 g/ L P. Polyandra leaves extracts concentrations.
Kinetics model
The corrosion reaction is a heterogeneous reaction which is composed of anodic and cathodic reactions at the same or different rate. It is on this basis that the data kinetic analysis is considered necessary.
For first order kinetics,
where w i is the initial weight of the mild steel, wt is the weight after time, t, and k1 is the first order rate constant, which has a unit of h-1.
Integrated first order is given as:
From the plot of In (w1 - wt) against t (h), k1 was estimated as the slope, while intercept as Inwt. The rate constant was calculated from its slope, as reported by the previous investigators [27]. The linear variation and slope, k1, confirm a first order reaction kinetics with respect to mild steel corrosion in a 1 M HCl solution, in the inhibitor presence. Figs. 11 and 12 show that the plots slopes have negative values and good correlation coefficients (R2) with obtained linear plots, which proofs that the reaction followed first order kinetic model, in line with Eddy et al. [28] findings that investigated the corrosion inhibition efficiency of ethanol extract of mango peel waste on mild steel.
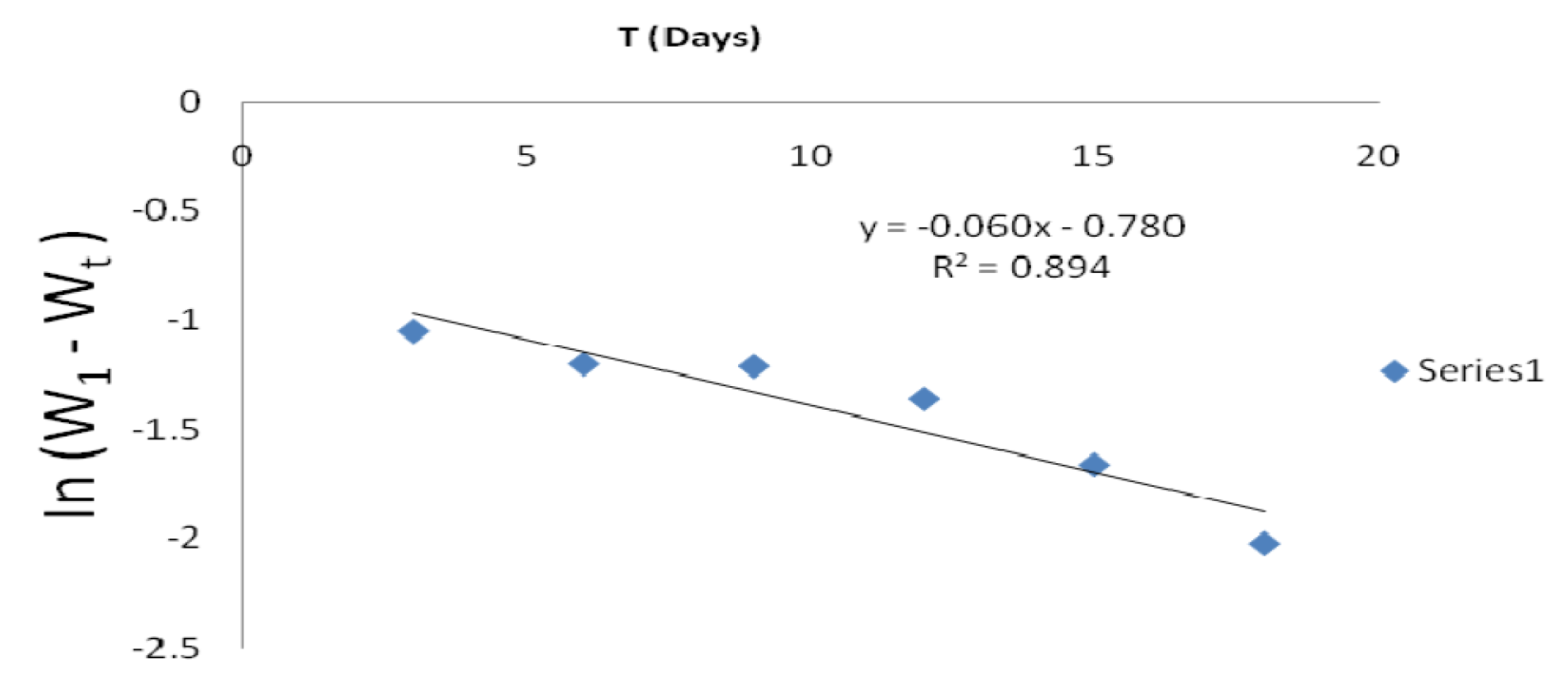
Figure 11 First order kinetic plot of mild steel weight loss against immersion time, in an uninhibited 1 M HCl solution.
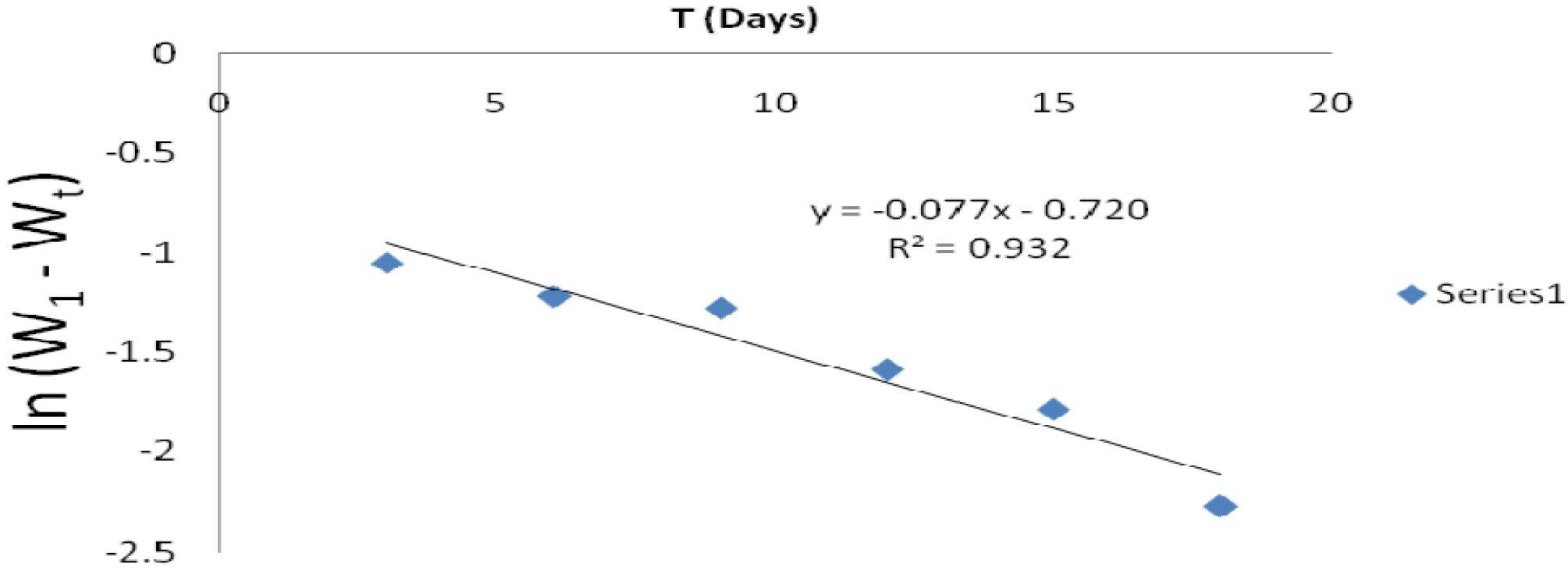
Figure 12 First order kinetic plot of mild steel weight loss against immersion time, in various P. polyandra leaves extracts concentrations, in a 1 M HCl solution.
Electrochemical studies
The electrochemical method of corrosion studies is the most reliable to give information about the mechanism of a corrosion process, and it is not a time consuming method, unlike the gravimetric method. Table 8 shows the parameters obtained from the electrochemical studies of P. polyandra leaves extract as corrosion inhibitor for mild steel in a 1 M HCl solution [29].
Table 8 Electrochemical parameters obtained from the P. polyandra leaves extracts electrochemical studies.
| Inhibitor concentration (g/L) | Ecorr (mv) | Icorr (µA/cm2) | βa | βc | C.R (mmpy) | I E (%) | Chi square |
|---|---|---|---|---|---|---|---|
| Uninhibited | -479.23 | -1.518 | 153.28 | 102.04 | 17.63 | NA | 52.87 |
| 0.1 g/L | -627.11 | -191.90 | 54.89 | 756.44 | 2.23 | 79.90 | 61.13 |
| 0.3 g/L | -589.45 | -13.13 | 328.72 | 1.565 | 0.15 | 88.49 | 195.72 |
| 0.5 g/L | -558 | -1.76 | 92.737 | 156.29 | 0.02 | 95.34 | 195.72 |
Electrochemical studies of mild steel corrosion inhibition using P. polyandra leaves extract, by Tafel electrochemical analysis, gave various corrosion parameters values for different inhibitor concentrations. Table 8 reveals that the leaves extract inhibitor altered the anodic beta (βa ) value (153.28) and the cathodic beta (βc) value (102.04) obtained from the uninhibited solution. Thus, the inhibitor acted as a mixed inhibitor [30].
From Table 8, it could be seen that the corrosion rate decreased significantly, from 17.63 mmpy of the uninhibited system, to 2.23 mmpy and 0.02 mmpy, at a concentration of 0.1 g/L and 0.5 g/L, respectively, of the leaves extracts. Fig. 13 represents the Tafel plot of the isolated leaves extract as corrosion inhibitor of mild steel in a 1 M HCl solution.

Figure 13 Tafel plot of isolated leaves extract as a corrosion inhibitor of mild steel in a 1 M HCl solution.
Scanning Electron Microscopy analysis (SEM)
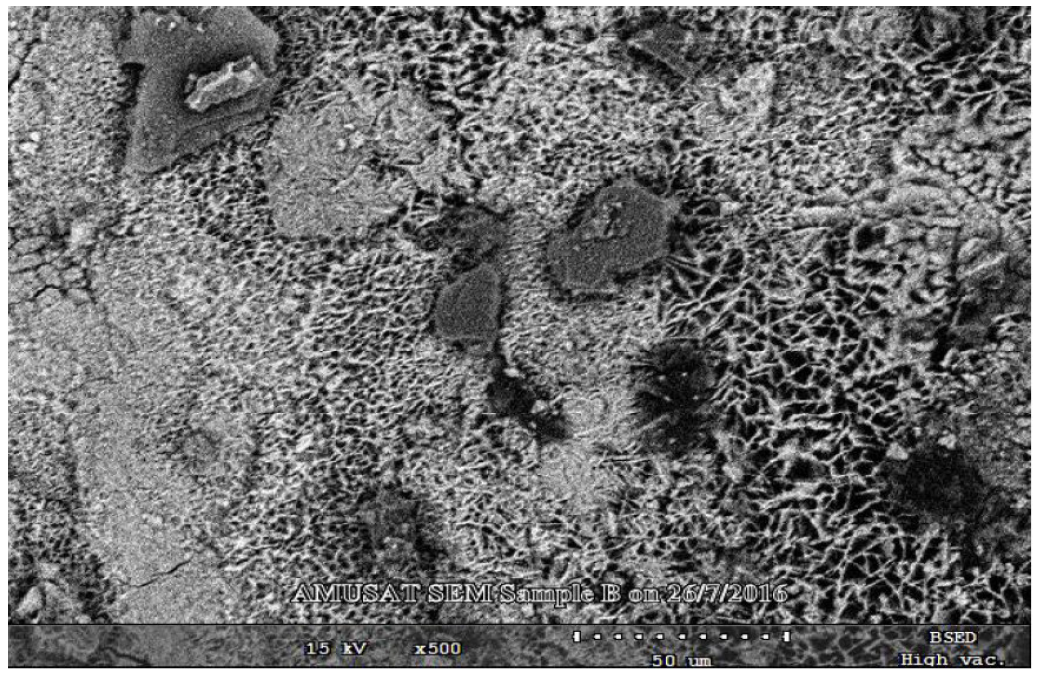
Scanning electron microscopy analytical technique revealed the visual physical morphological changes on the surface of mild steel that was separately immersed in uninhibited 1 M HCl solutions and in inhibited HCl solutions with different P. polyandra leaves extract concentrations. SEM images for mild steel immersed in an uninhibited 1 M HCl solution and in a solution with 0.5 g/L of leaves extracts, for 48 h, at 303 K, are shown in Figs. 14 and 15. The images revealed that, in the inhibitor absence, cracks resulted from a corrosion attack on the mild steel surface, while the cracks and corroded areas were reduced in the SEM images of the mild steel inhibited with various leaves extract concentrations. This is due to the formation of protective layers by the molecules inhibiting the mild steel corrosion [5].
The image in Fig. 14 shows many cracks due to the corrosion attack on the mild steel surface [10]. The image in Fig. 15 reveals no obviously crack occurrences. This is due to the presence of the leaves extract inhibitor that was adsorbed onto the mild steel surface, thus protecting it from corrosion attack [5, 10].

Figure 14 Mild steel SEM micrograph in an uninhibited 1 M HCl solution, after 48 hours of immersion, at a magnification of 500x.
Conclusion
In conclusion, ethanolic leaves extract of P. polyandra exhibited good inhibiting characteristics against mild steel corrosion in a 1 M HCl solution. This might be due to the carbonyl and hydroxyl groups presence in the leaves extract, which served as an adsorption site onto the mild steel surface. SEM analysis also confirmed the mild steel corrosion inhibition, in a 1 M HCl solution, by the P. polyandra leaves extract, showing its high inhibition potential. Both gravimetric and electrochemical methods showed that the leaves extract solution inhibition efficiency decreased with higher temperatures and increased with longer mild steel immersion periods in it. Langmuir adsorption isotherm best described the leaves extract adsorption onto the mild steel surface, which has followed an endothermic process. The first order kinetics also favored the P. polyandra leaves extract adsorption onto the mild steel surface.