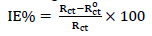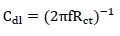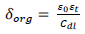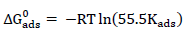Introduction
Metallic corrosion is affected by environmental factors (including moisture and T) and surface conditions, such as energy, exposed area, roughness and oxides stability, which are inherently related to the materials composition. Unanticipated failures (stream generator and pipeline stress corrosion cracking) and system shutdowns occur in oil and gas industries, at pipelines, liquefied natural gas terminals and refineries, due to corrosion, which results in monetary losses. When analyzing any method to control corrosion in industrial systems, operational processes should comprise factors such as coatings durability, maintenance schedule, service life, sustainability and life cycle costs.
The selection of the most effective corrosion control system is important. Corrosion protection systems include galvanizing, painting, coating, and the use of CI, such as organic, inorganic, volatile, adsorption-type, anodic, cathodic, mixed and environmental friendly inhibitors. CI performance is mainly related to the molecules physicochemical properties, such as their electronic structure, electron density at the donor atom and functional group. Pyridine and its derivatives are classified as accepted CI. The influence of numerous functional groups on pyridine, and their derivatives, capability as CI for various metals and alloys in acidic/alkaline media, has been reported in literature 1-12. The heterocyclic compound is adsorbed onto the metallic surface through their active sites. The inhibitor adsorbed protective layers shield the metallic surface from direct contact with corrosive ions 13-23.
The present paper studied the CI of MS in H2SO4 by CP only and CP + KI, employing Ec methods such as EIS. It discussed the complex-plane impedance Nyquist and Bode plots for MS in 0.5 M H2SO4, without and with different C of CP, and it also described KI synergistic effect at 308 K. SEM and AFM techniques were used to analyze the SM of MS.
Experimental analysis
Specimen preparation
Uniform surfaces of MS specimens were prepared with a grinding machine using emery paper of different grades (220, 400, 800 and 1000). MS was employed as WE. For the EIS experiment, the WE exposed area was of 1 cm2.
Ec technique
Ec investigations were done by an CHI 760D model Ec work station. EIS experiment was performed with an AC signal of 1 mV amplitude, at OCP, and at a frequency range from 100 KHz to 10 MHz.
SM
The SM of MS coupons was studied using a Ziess S-3700 N SEM and Nanosurf Naio AFM (both from Germany). The MS surface area of 1 cm2 was smoothened with 150, 600 and 2000 grade emery paper, and then cleaned with distilled water and acetone. MS was exposed to H2SO4 without and with 10-1 M CP only and CP + KI, at 298 K, for 6 h, and then it was washed and dried before surface examinations.
Results and discussion
EIS
The CI of MS was investigated by EIS method. Fig. 1 shows impedance plots for MS in H2SO4 without and with CP only and CP + KI, at 308 K. Nyquist plots show that the depressed semi-circle increased with higher C of CP, indicating that MS corrosion was largely controlled by a charge transfer mechanism. This kind of behavior is a common feature of solid electrodes, which causes frequency dispersion, roughness and inhomogeneities on the solid electrode surface.
EIS parameters, such as Rct, Cdl and IE (%), are shown in Table 1. IE (%) was evaluated by using the following relation 24:
where Ro ct denotes Rct of the solution without inhibitor. Cdl was determined from the equation given below:
The Bode plots (Fig. 2 a-d) show that θmax values are related to the electrode (. Low θmax values correspond to high (. Table 1 shows that θmax in H2SO4 was about 26.3, and it gradually increased upon CP + KI addition, which suggests that ( decreased with the inhibitor. θmax observed upon I ions addition indicates that the system was approaching an ideal polarized electrode.

Figure 2: Bode plots of MS in 0.5 M H2SO4, at 308 K, with various C of: (a) and b) CP; (c) and (d) CP + KI.
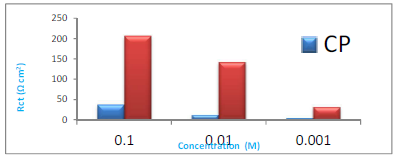
Table 1 reveals that there was increase in Rct value with higher CP’s C, from 10-3 to 10-1 M (Fig. 3). In contrast, there was a decrease in Cdl value with higher CP’s C. Due to the synergistic effect of I ions and the rise in the inhibitor’s C, the interface Rp increased, because of CP + KI molecules adsorption onto the MS surface.
Table 1: Demonstration of EIS data on MS with various C of CP and CP + KI in 0.5 M H2SO4.
| Additive | Conc. (M) | Rct (Ω/cm2) | Freq. (Hz) | Cdl (F/cm2) x 10-4 | IE(%) | θ | θmax |
|---|---|---|---|---|---|---|---|
| H2SO4 | 0 | 2.404 | 11.91 | 55.62 | - | - | 26.3 |
| CP | 10-1 | 39.561 | 1.738 | 2.316 | 93.92 | 0.939 | 49.1 |
| 10-2 | 13.192 | 4.542 | 2.658 | 81.77 | 0.817 | 47.6 | |
| 10-3 | 5.156 | 8.071 | 3.826 | 53.37 | 0.533 | 42.3 | |
| CP + KI | 10-1 | 206.179 | 1.43 | 5.39 | 98.83 | 0.988 | 59.3 |
| 10-2 | 142.461 | 2.55 | 4.38 | 98.31 | 0.983 | 55.8 | |
| 10-3 | 32.315 | 2.55 | 19.32 | 92.56 | 0.925 | 49.9 |
( by CP and CP + KI molecules allowed for CI 25. The following equation shows the correlation between Cdl and the protective layer thickness.
where εo and εt denote vacuum and relative dielectric constants, respectively. Cdl values were reduced, due to a decrease in the local dielectric constant or to an increase in the electrical double layer, caused by CP molecules’s adsorption onto the metal/solution interface, and by the water molecules gradual replacement by the inhibitor on the MS surface. This resulted in lower MS dissolution 26, confirming that CP effectively retarded MS corrosion process in H2SO4.
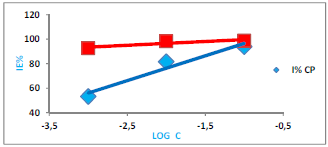
Fig. 4 shows the relation between CP’s IE (%) and Log C, at 308 K, and that IE (%) increased with CP and CP + KI’s higher C, from 10-3 M to 10-1 M.
CP’s IE (%) values were 53.3, 81.7 and 93.9%, at 10-3, 10-2 and 10-1 M, respectively. However, they were 92.5, 98.3 and 98.8%, at 10-3, 10-2 and 10-1 M, respectively, upon I ions addition accordingly 27,28. Due to the large ionic radius and high hydrophobicity, I ions are easily participated in the adsorption process on the MS surface through a synergetic effect, which caused the corrosion inhibition 29-32. CP seems to be an excellent inhibitor in 0.5 M H2SO4, and its potential was enhanced by KI, since it achieved an IE (%) of 98.8%. Similar outcomes have been reported in literature 33,34.
Adsorption isotherm
( parameters determined from EIS measurements are listed in Table 1, and they were used to identify the best adsorption isotherm. Ec measurements confirmed the best fitted Langmuir’s adsorption isotherm, for which the following relation has been used 35:
Fig. 5 shows that the plot between C/θ and C of CP and CP + KI in 0.5 M H2SO4, at 308 K, gave a straight line.
It is evident from the outcomes that linear R2 was 1, which implies that the adsorption mechanism of CP and CP + KI molecules onto the MS surface obeyed Langmuir’s isotherm 36 (Table 2).
Table 2: Karl Pearson’s coefficient of CP and CP + KI adsorption isotherm in 0.5 M H2SO4.
| Organic inhibitor | Adsorption isotherm | R2 |
|---|---|---|
| CP | Langmuir´s | 0.9999 |
| CP + KI | Langmuir´s | 1 |
Standard ΔGads of CP and CP + KI is related to Kads, and it can be evaluated by the following equation 37:
where R represents the universal gas constant and 55.5 is the molar C of the solvent (H2O). CP and CP + KI’s ∆G ads 0 values were negative (-27.97 and -34.13 kJ/mol, respectively), which indicates that their adsorption onto the MS surface was a spontaneous process that occurred through a chemical bond (chemisorption) 38 (Table 3).
Table 3: Adsorption parameters for MS surface corrosion in H2SO4 with inhibitors, at 308 K.
| Inhibitor | Intercept | K (L/mol) | ∆𝐆 𝐚𝐝𝐬 𝟎 (kJ/mol) |
|---|---|---|---|
| CP | 1 x 10-3 | 1000 | -27.97 |
| CP + KI | 9 x 10-5 | 11,111.1 | -34.13 |
SEM
SEM images of MS specimens after immersion in H2SO4 with 10-1 M CP and CP + KI show a smoother surface (Fig. 6a and b). This is because the inhibitor molecules hindered MS dissolution process, by developing a barrier layer on its surface, which retarded the corrosion process.
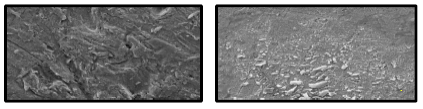
Figure 6: SEM images of the MS surface in 0.5 M H2SO4 with: (a) 10-1 M CP; and (b) 10-1 M CP + KI (magnification × 2000).
Hence, CP protected MS against corrosion in H2SO4 solutions. In addition, from the images, one also concludes that I- ions played an important role in CI, through their participation in the adsorption process, which enhanced MS protection 39,40.
AFM
AFM image in Fig. 7(a) shows that, upon CP addition to H2S04, some small, even and compact particles, which are orderly scattered on the MS surface, covered it almost completely.
On the other hand, AFM image in Fig. 7b shows that, with CP + KI, the MS surface became more flat, uniform and homogeneous, which reveals that the inhibitor offered an appreciable resistance to corrosion, due to I- synergetic effect.
The MS surface ( in blank H2S04 was up to 1102.8 nm, while it decreased to 392.9 nm upon CP addition, and was further reduced to 259 nm with CP + KI (Table 4).
Similar results were reported in literature for organic inhibitors in acidic media 41,42. Therefore, MS CI took place due to the formation of a protective layer by CP and CP + KI, through the adsorption of their molecules onto the alloy surface. The three-dimensional AFM images of the MS surface, in H2S04 with CP and CP + KI, are shown in Fig. 8a and b.
Conclusion
The outcomes of the present work revealed that the studied inhibitors are indeed very promising, with excellent ability to hinder MS corrosion process in H2S04. EIS results showed that, upon the addition of CP and CP + KI to H2S04, the protective film developed on the metal/electrolyte interface caused an increase in Rct, and a decrease in Cdl. It was also confirmed that the inhibition mechanism was linked to CP and CP + KI adsorption, which obeys Langmuir’s isotherm. The values from SM ( studies in the solution with inhibitor were much lower than those in the blank one. This means that CP and CP + KI are effective organic CI.
Authors’ contributions
Neelu Dheer: was in main charge of this research, having performed experiments and analyzed experimental data; discussed results and helped analyzing them. Amarpreet K. Kalra: was in main charge of this research, having performed experiments and analyzed experimental data. Darshan Singh: discussed results and helped analyzing them. Sanjeev Kumar Ujjain: edited the paper. Preety Ahuja: edited the paper. Gurmeet Singh: reviewed and edited the paper. M. Ramananda Singh: conceived the idea and supervised the whole project; prepared the paper. Rajni Kanojia: conceived the idea and supervised the whole project; prepared the paper; edited the paper.
Abbreviations
AC: alternating current
AFM: atomic force microscope
C: concentration
Cdl: double layer capacitance
CI: corrosion inhibitor/inhibition
CP: 3-carboxypyridine
Ec: electrochemical
EIS: electrochemical impedance spectroscopy
H2SO4: sulphuric acid
IE(%): inhibition efficiency
Kads: adsorption equilibrium constant
KI: potassium iodide
Log C: logarithm concentration
MS: mild steel
OCP: open circuit potential
R2: correlation coefficient
Rct: charge transfer resistance
Rp: polarization resistance
SEM: scanning electron microscopy
SM: surface methodology
T: temperature
WE: working electrode