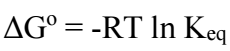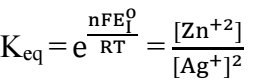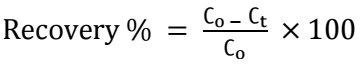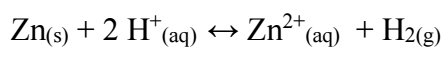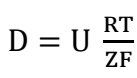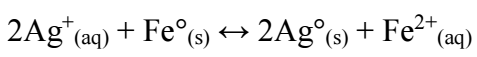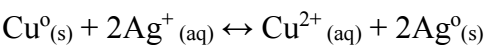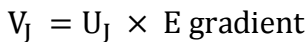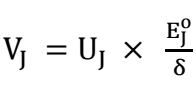Introduction
Ag is a precious metal, which is used in many fields, such as jewelry, dentistry, photography, optics, electroplating, solar panels and electronic components. For decades, Ag has been used to make photographic and X-ray films, due to its light-sensitive unique properties. Despite the emergence of new advanced digital imaging equipment, many countries with arising economies still use analogue X-ray equipment 1. Ag properties make it an ideal catalyst in oxidation reactions, e.g., formaldehyde production from methanol and air 2.
Presently, almost 80% Ag global production comes from Earth’s crust, as a secondary product of Cu, Zn and Pb mines production. The natural Ag mines provide the remaining 20% 3. Ag world production has become insufficient 4,5. Therefore, to meet Ag increasing demand, its recycling is paramount in economical and practical terms.
It has been reported "that about 40 to 50 percent of the silver production was consumed in radiography and photographic films, and 25 percent of the silver demand is obtained by recycling silver wastes, including photographic ones" 6. The amount of Ag that waste X-ray films contain is dependent on a number of factors, including the film's age, the company that produced it, and whether it has already been developed or not 7. To sum it all up, it was stated that "10 pounds of medical x-ray film usually contains about one oz t. of Ag" 8.
Ag is not annihilated in the photographic process and it can be recovered and reused. For Ag recycling from X-ray films, there are several different treatment processes (5), such as cementation, i.e., metal replacement. Apparently, this word was derived from the Spanish term cementación, which means precipitation 9.
Cementation is an electrochemical process that differs from electrolytic deposition (where the electrons are supplied from an external power supply) and from electroless plating (where the oxidation of an organic additive occurs). It is a simple process for metallic removal/recovery from a solution, through a spontaneous electrochemical reaction between a cementing metal and the ion of the metal to be separated. The significant advantages of this process include 10: the capability of removing ions of very low concentrations from large volumes of diverse electrolytic solutions, where other methods are much more expensive or inapplicable, simple control requirements, low energy consumption is because the majority of the energy used is to agitate the treated waste solution, and the process is typically carried out at temperatures well below 373.15 K, and recovery of valuable metals such as Ag and Cu with high purity.
However, 11,12 reported that the cementation process has some drawbacks, such as lower processing rates, the need for expensive cementing metals, when they are used in powder form, and metallic dissolution in the media, mostly at low pH values, which may create new impurities. Therefore, to avoid contamination by other ions, it is required to use a nontoxic metal that is already present in the treated solution.
The aim of the current research was to recover Ag from solutions and solid wastes. To accomplish this target, the following three research investigations were done: The first was to investigate the effect of operating parameters on Ag recovery from its nitrate solution to determine the most significant parameters. The second is performing experiments to recover Ag from a solution containing Cu and Ag and from a solution containing Cu, Pb, and Ag. The third, as a practical application, is to recover Ag from discarded X-ray films.
Experimental work
Set up
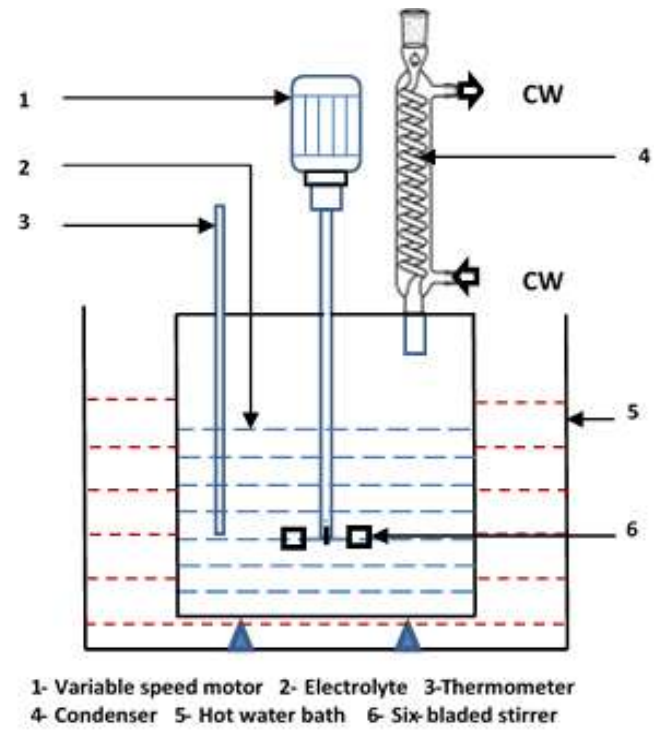
Experiments were carried out using the set-up shown in Fig. 1, which consisted of a cylindrical glass container with an internal diameter of 10.5 cm and capacity of 1 dm3. At the center line of the container, a turbine impeller of 4 cm outer diameter, made of 316 SS, was positioned 4 cm above the bottom of the container. The impeller was connected to a variable-speed motor via a 316 SS shaft of 0.5 cm diameter. Both the impeller and its shaft were covered with epoxy resin so as to be isolated from the processed solution to avoid their participation in the cementation process. A thermometer was placed inside the container to adjust and follow the temperature of the treated solution. The container and its contents were immersed in a rectangular water bath that enabled manual adjustment of its temperature and, consequently, the temperature of the processed solution. To maintain the water content of the treated solution, a vertical water condenser was mounted at the top of the container to condense the evolved water vapor.
Materials
All materials used in the experimental work were laboratory-grade (from El-Gomhouria Company for Trading Chemicals and Medical Appliances, Cairo, Egypt).
Methodology
Before the start of a set of experiments, both surfaces of the precipitant metal sheet were degreased with acetone, cleaned from any adhered oxides with emery paper of grit size 100, etched in diluted HCl, to remove any remaining oxides, thoroughly washed with distilled water, and dried.
A solution of a pre-specified Ag+ concentration, C, was prepared by dissolving the proper amount of AgNO3 in freshly distilled water. The initial solution pH was adjusted by adding drops of either 0.1 M HNO₃ or 0.1 M NaOH. The pH was measured using a JENWAY 3505 pH meter, with 0.02 accuracy.
In each run, 500 mL the synthetic solution were placed in the cylindrical glass container. Temperature, pH and impeller speed were adjusted to the required values. A fixed mass of the cementing metal was added to the stirred solution. At specified time intervals, samples were withdrawn by a pipette, for measurement of their pH to readjust the pH of the processed solution. Two mL of the sample were then diluted with demineralized water, and C of Ag was measured by AAS (Perkin Elmer 2280).
Effect of operational parameters on Ag recovery
The studied parameters and their values are given in Table 1.
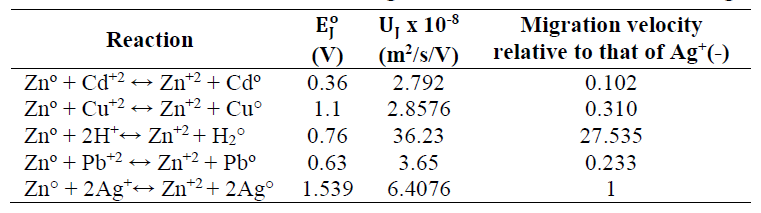
Eq. (1) describes the cementation reaction between Ag+ and Zn. Basically, the system can be visualized as consisting of a set of short-circuited electrolytic microcells.
The standard cell potential of reaction (1), (E_I^o), is 1.539 V. If reactants and products are in their standard states, ΔGo is:
where n is number of transferred electrons and F is Faraday’s constant. Also,
where R is the universal gas constant. From Eqs. (2) and (3):
where (Ag+) and (Zn+2) are ions molar C at the equilibrium state. At 298.15 K, Keq = (〖(Zn〗^(+2)))/((〖Ag〗^+ )^2 ) = 1.076 x 1052, which means that reaction (1) was thermodynamically favored to go on to the right direction. However, it is heterogeneous in nature, being controlled by either a reaction at the cementing metal surface or by mass transport. Since E_I^o > 0.36, mass transfer controls reaction (4) 13. The percent recovery of Ag was calculated using equation (5).
where Co and Ct are the C (mg/dm3) of the assigned cation, initially and over time (t), respectively.
Effect of Zn:Ag+ molar ratio
The effect of Zn (in the form of a sheet) to Ag+ molar ratio on Ag recovery is shown in Fig. 2.
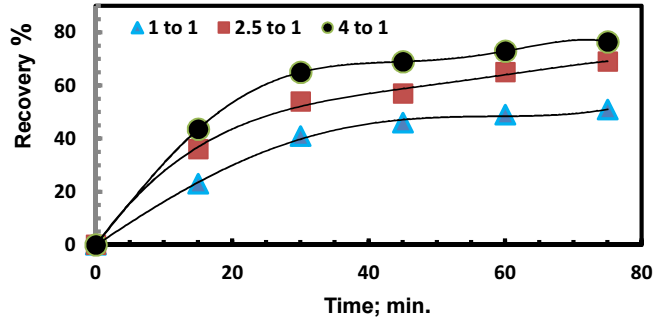
Figure 2: Ag recovery for different Zn:Ag+ ion mole ratios. (Co)Ag+ = 500 mg/dm3; pH = 5; rpm = 300; T = 303 K.
Experiments were performed by changing the molar ratio from 1:1 to 4:1, while other operating parameters were kept at constant values. In all cases, in the first 30 min, Ag recovery was higher than afterwards. A similar behavior was reported by others 14,15, which was attributed to the formation of a cemented Ag layer on the Zn surface, which hindered Ag+ ion mass transport.
On the other hand, Ag recovery increased with higher Zn:Ag+ ion molar ratios. This was simply due to Zn sheet larger surface area, which enhanced its contact with Ag ions contact.
Effect of pH
The Pourbaix diagram for Ag-Zn-H2O 15 can be used to predict which species is thermodynamically stable at a given E and pH, but it provides no information on the kinetics of the reaction under consideration. The diagram shows that Ag(s) is stable when the pH of the solution is between 0 and 14 and at almost all the E values where water is stable. Zn2+ is stable at a pH value of 5.65, whereas ZnO(s), which is insoluble in water, is stable at pH values greater than 5.65. Based on this information, pH values of 1, 3 and 5 were employed, to elucidate its effect on Ag recovery. Experimental results are shown Fig. 3.
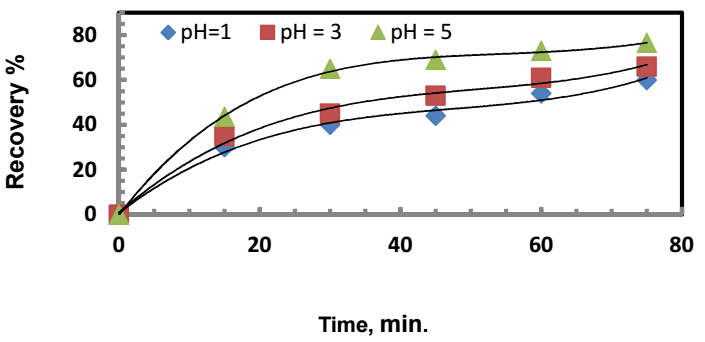
Figure 3: Solution pH effect on Ag recovery. Zn:Ag+ = 4:1; (Co)Ag+ = 500 mg/dm3; rpm = 300; and T = 303 K.
The percent recovery of Ag was found to decrease as pH decreased from 5 to 1. This is due to the increased competition between Ag+ and H+ ions for discharge sites on the Zn surface owing to the following reaction:
which proceeds to a greater extent at low pH values, and as a consequence, the Zn surface area available for Ag cementation is decreased.
Effect of stirring speed
Stirring speed effect on Ag recovery was investigated under the following operating parameters: Zn:Ag+ mole ratio = 4:1; (Co)Ag+ = 500 mg/dm3; pH = 5; and T = 303 K. The results are shown in Fig. 4.
Ag recovery rate increased with higher stirring speeds. The same behavior was previously observed 14,16 and 17, when Ag+ was cemented onto Cu. The increase in the recovery rate is attributed, on the one hand, to the increased convective mass transport of Ag ions in the bulk solution and, on the other hand, to the mechanical breakage of the cement layer formed on the Zn surface, thus freeing some of the active sites for the cementation process to proceed.
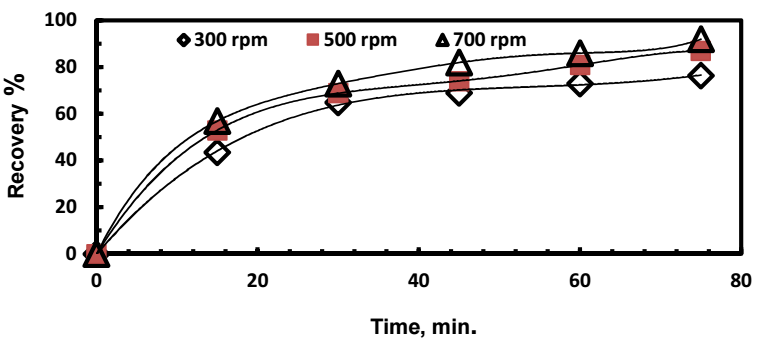
Figure 4: Stirring speed effect on Ag recovery: Zn:Ag+ = 4:1; (Co)Ag+ = 500 mg/dm3; pH = 5; T = 303 K.
Effect of temperature
The effect of temperature was investigated at 303, 318, and 333 K. In these experiments, the other parameters were held constant at the values given in Fig. (5). The cementation rate increased with increasing temperature. This increase is due to the increased diffusion of Ag ions across the laminar boundary layer surrounding zinc surface. The ion diffusion coefficient, D, can often be estimated from Eq. 7, which correlates a direct relation between D and T 18.
where U and Z are the ion mobility and charge, respectively.
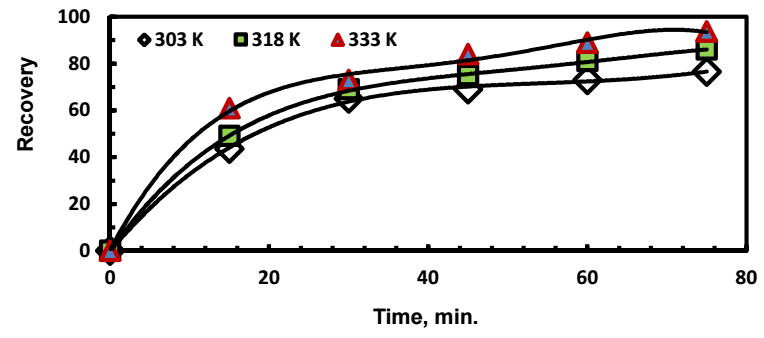
Figure 5: Effect of solution temperature on Ag recovery. Zn:Ag+ 4:1; (Co)Ag+ = 500 mg/dm3; pH = 5; rpm = 300.
Ag recovery by iron
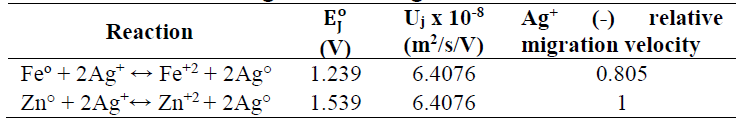
Due to its availability and low cost, Fe scraps can be used to recover Ag. The reaction is:
The standard cell E of this reaction (E_II^o) is 1.239 V. Hence, according to Eq. 4, at T = 298.15 K, Keq = (〖(Fe〗^(2+ )))/((〖Ag〗^+ )^2 ) = 7.748x1041, which means that reaction (8) is thermodynamically favored to go in the right direction. The comparison between E_I^o of reaction (1) and that of reaction (8) indicates that, for Ag recovery, Zn was stronger than Fe (Fig. 6).
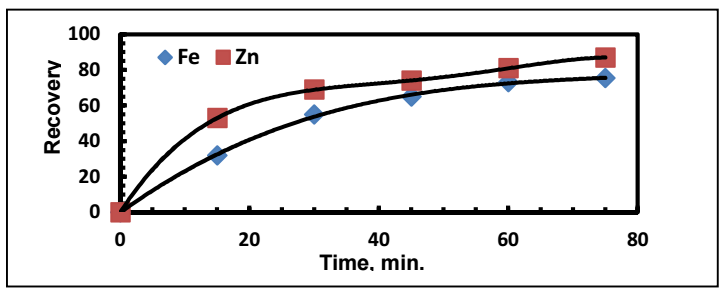
Figure 6: Comparison between Zn and Fe use for Ag recovery: Zn:Ag = 4:1; Fe:Ag+ = 4:1; (Co)Ag+ = 500 mg/dm3; pH = 5; rpm = 300; T = 303 K.
Copper can also be used for the same purpose, and the reaction is
At standard conditions, E_III^o value of reaction (9) is 0.459 V, and although being lower than that of reactions (1) and (8), it still enables Cu to displace Ag from its solution. There are at least three Ag recovery options that allow for tailoring the process to a specific set of needs. As an example, an Ag solution tainted with Cu may be cemented by it. Thus, the latter replaces the former, being cemented afterwards by Fe or Zn for Cu recovery.
Ag and Cu sequential recovery
As aforesaid, 80% of global Ag production from the earth's crust is produced as a byproduct of Cu, Zn, and Pb mine production. Accordingly, an experiment to recover Ag from a solution containing Ag and Cu ions was performed, in which Zn was the cementing metal. The experiment's operating variables and results are shown in Fig. 7. It is noteworthy that, before the cementation reaction onset, the Cu2/Ag+ ion molar ratio in the solution was about 1.7, which means that Cu2+ was the dominant cation.
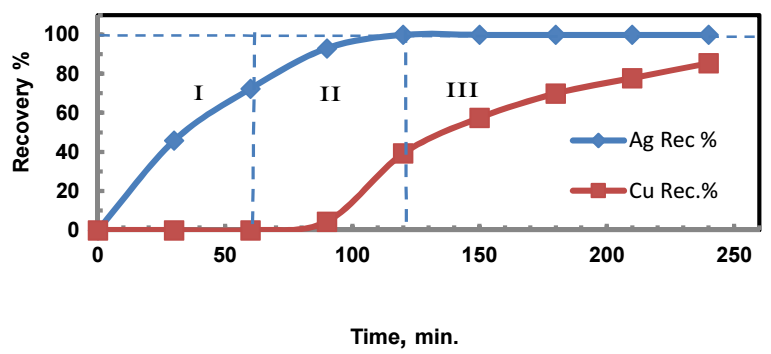
Figure 7: Ag and Cu sequential recovery: (Co)Ag+ = (Co)Cu+2 (500 mg/dm3); Zn:Ag+ = 3:1; Zn:Cu+2 = 1.5:1; T = 303 K; pH = 5; rpm = 500.
Fig. 7 illustrates that, after 60 min, 72.5% Ag was recovered, while all Cu+ remained in the solution. After 90 min, Ag recovery rose to 93%, while that of Cu was 4.4%. After 120 min, Ag recovery reached 100%, and that of Cu was 39.3%. After 240 min, Cu recovery rose to 86%. The results displayed in the figure show that Ag and Cu sequential recovery occurred in three distinct regions: Ag was selectively recovered in region I; Ag and Cu combined recovery happened in region II; and Cu was recovered in region III. Ag selective recovery in region I may be explained by considering that: both Ag+ and Cu+2 ions have different C, at the interface between the convective and laminar boundary layers, which are directly present at the Zn surface. As aforementioned, Cu+2/Ag+ ions molar ratio was 1.7. Therefore, it was expected that Cu+2 would precipitate before Ag+, if the mass transport was only due to C gradient. Actually, each ion found itself under a different electric driving force (zFEJ) (where EJ is the cell E between an ion J and Zn), which moved them towards the Zn surface, with different migration velocities. For an ion J, the migration velocity, VJ, assuming standard conditions, is defined as:
where U_J is the ion mobility. Then, at standard conditions,
where δ is the laminar boundary layer thickness.
At standard conditions, Ag+ ion absolute mobility (U_(Ag+) = 6.4 x 10-8 m2/s.V) is larger than that of Cu2+ (2.926 x 10-8 m2/s.V). Hence, Ag+ migration velocity (U_(Ag+) x (E_I^o)/δ) is larger than that of Cu2+ (= UCu2+ x (E_III^o)/δ).
Therefore, Ag+ initially covered all the Zn surface active sites, and prevented Cu+ ion from approaching them. After the initial 60 min. 72.5% Ag were recovered, and some free active sites became available for Cu+ ion reduction. This is why Cu was co-separated with the remaining Ag, in region II. After the first 120 min, complete recovery of Ag was fulfilled, and Cu was only recovered in region III.
Cu, Pb and Ag sequential recovery
To meet the challenge of handling a solution containing more than two metal ions, Cu, Pb and Ag sequential recovery from their nitrate solution was carried out. The metals sequential recovery is shown in Fig. 8.
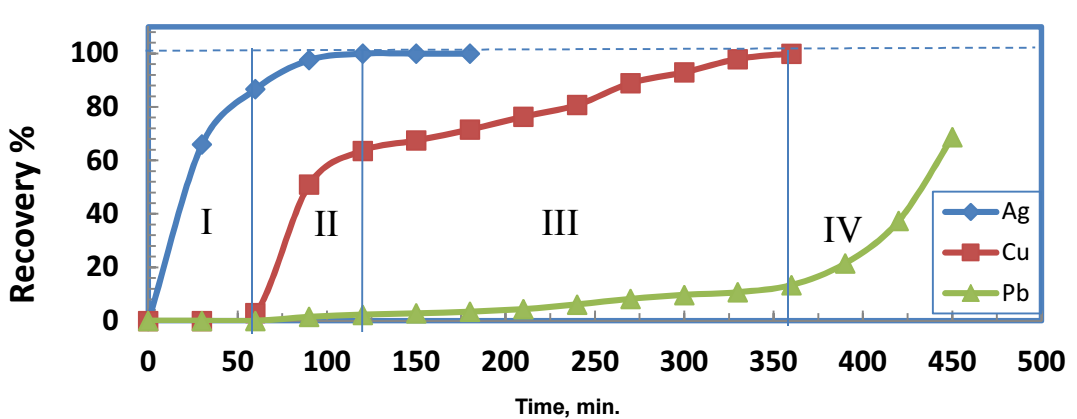
Figure 8: Ag, Cu, and Pb sequential recovery. (Co )Ag = (Co )Cu = (Co )Pb = 500 mg/dm3; Zn/Ag = 3:1 Zn/Cu = 1.5:1; Zn/Pb =1:1; T = 303 K ; pH = 5; rpm = 500.
There are four regions. In region I, from 0 to 60 min, 87 and 3% Ag and Cu were separated, respectively. At the end of region II, 120 min from the start, Ag was completely detached, while 64 and 2.5% Cu and Pb were separated, respectively. Cu was completely separated by the end of region III, 360 min from the start, along with 13.5% Pb separation. In region IV, only Pb was separated.
The interpretation of the results from this experiment may be like the one for those of Ag and Cu sequential separation. However, it is noteworthy that, although Pb+ ion mobility was greater than that of Cu+ ion, Cu was separated before Pb. This was because standard E of the reaction between Zn and Pb+ ions was smaller than that of the reaction between Zn and Cu+, as shown in Table 2. Thus, Cu+ migration velocity was greater than that of Pb.
To clarify the role played by ion migration velocity in metal sequential recovery from a solution containing mixed metallic ions, the results presented by some authors who used other techniques were considered. Cu, Cd, and Zn could be sequentially separated according to the written order, based on the results of laboratory experiments performed under controlled E electrolysis for treating a mixed metal solution 19. Using a microbial bio-electrochemical system, Cu, Pb, Cd, and Zn could be sequentially recovered 20. In these two studies, the authors mentioned that Zn was present in the two treated solutions. Therefore, Zn can be used as cementing metal, whenever the two solutions are treated by cementation. Table 2 shows that the ions migration velocities in descending order were V_(Cu^(+2) ) > V_(Pb^(+2) ) >V_(Cd^(+2) ). This reveals the sequential separation of Cu, Pb, and, finally, Cd. As for Zn, its quantity increased in the treated solution. Zn can be recovered by cementation, using a metal that is more active than it, or by any other suitable method.
The effect of H+ concentration, i.e., the processed water solution pH, on Ag recovery rate by cementation, can also be interpreted according to the role played by the ion migration velocity towards the Zn surface. Compared to other ions in Table 2, H+ holds very high mobility in diluted water solutions. Fig. 3 shows that, after 15 min cementation, Ag recovery was about: 22% at pH 1 and (H+) = 0.1 mol/dm3; and 45% at pH 5 and (H+) = 0.01x10-3 mol/dm3. This decrease in (H+) means that the chance for Ag+ to reach the Zn surface was considerably increased.
The results presented in Fig. 6 can also be read considering the ion migration velocity effect on Ag recovery by Zn and Fe. Table 3 clearly shows that Ag+ ion migration velocity, when using Zn, is greater than when using Fe, which leads to an increase in Ag recovery for the same operating time.
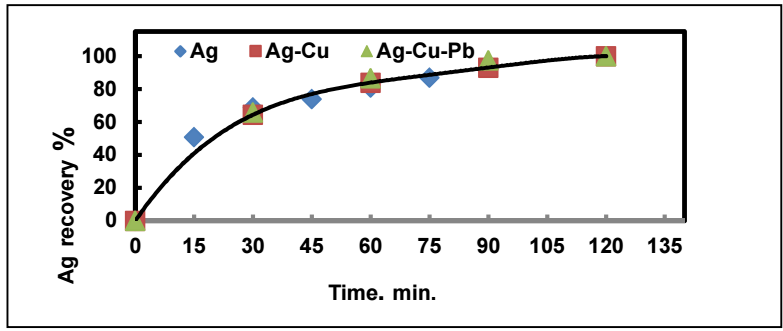
Herein, the cation migration velocity effective role in the process of metals recovery from their aqueous solutions, by cementation, was clarified. However, a question on the sequential separation, by cementation, of a group of metals from the same mixed-metal solution, remains. Does the presence of multiple cations in the same solution influence the migration velocity of any of them? The answer to this question is given by Fig. 9, which shows Ag recovery rate from solutions containing Ag+, Ag+ and Cu+ ions, and Ag+, Cu+, and Pb+ ions. Ag recovery rate was practically unchanged by other ions associated with Ag+ ion in the solution.
Ag recovery from spent x-ray films
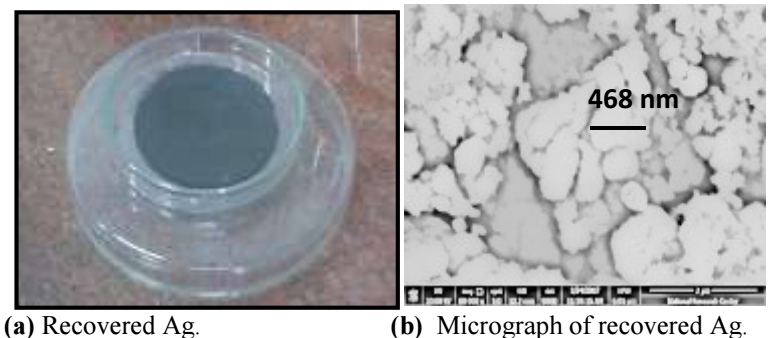
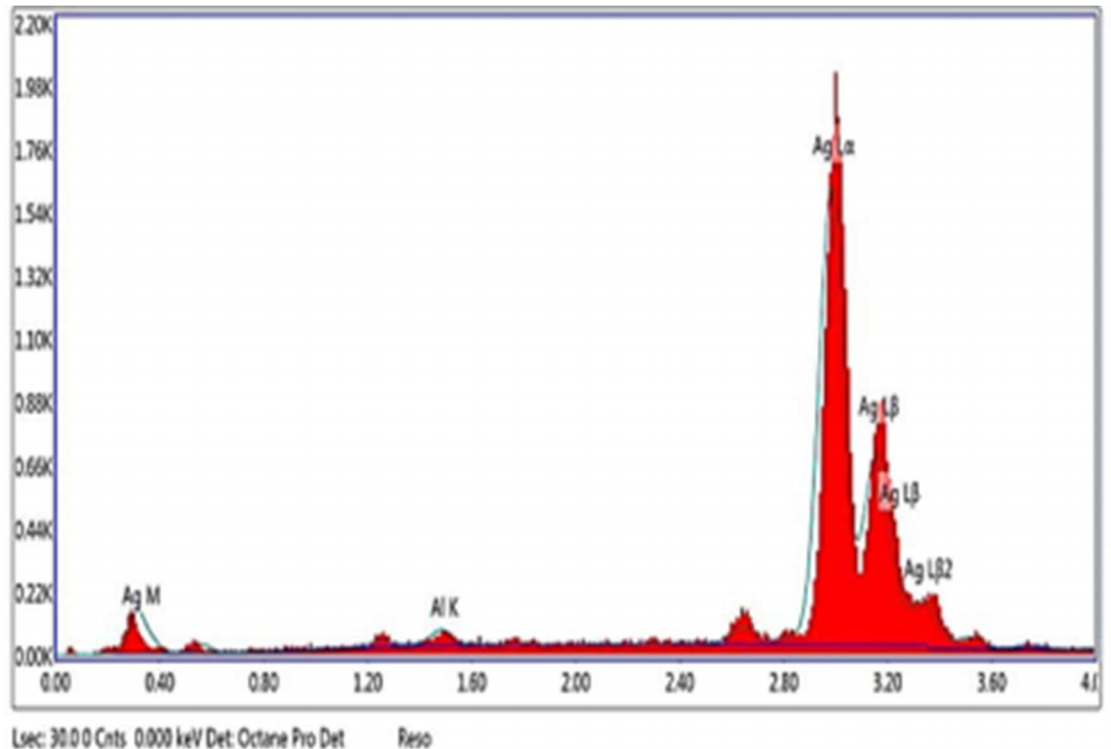
Ag recovery from spent diagnostic x-ray films was performed as a practical application of this study. The recovery was done by taking the following steps: the used X-ray films were washed with distilled water, wiped with cotton impregnated with ethanol, dried in an oven at 40 ºC, and finally cut into pieces of 2 x 2 cm2. The pieces were placed in a glass beaker, to which an Ag-extracting solution (0.5 M HNO3) was added. The contents were heated up to 50 ºC, and stirred at a rate of 500 rpm. The process was completed when the film black layer was removed, and the plastic sheet became transparent. The solid residues were separated from the clear solution, by filtering its contents. Ag was recovered by cementation, using a Zn sheet. Figs. 10-a) and b) are photos of the waste before and after treatment, respectively. Figs. 11 -a) and b) show the deposit separated by filtration, and its micrograph, respectively. The deposit color was black, because it had tiny particles 21. EDX elemental analysis is shown in Fig. 12.
Conclusions
Ag recovery by cementation from its monometallic, bimetallic, and trimetallic nitrate solutions was successfully accomplished using a simple stirred reactor. The recovery rate is higher within the first 30 minutes after the start as compared to that attained during later, similar time periods. The rate increased with a higher Zn/Ag molar ratio, rotational speed, and T. It decreased with a higher acidity. Ag cementation on Zn was controlled by Ag+ mass transport towards the Zn surface.
Ag, Cu, and Pb were sequentially recovered from solutions containing an initial C of 500 mg/dm3 each. For metallic sequential recovery from a mixed metal solution by cementation, the ions migration velocities determined the order by which they were separated.
Ag recovery from spent X-ray films was achieved as a practical application of this study. The recovered Ag purity was 98.3%.
Authors’ contributions
I. A. Khattab: conceptualization; experimental methodology; investigation; data curation and visualization; wrote the original draft. S. I. Hawash: performed some experimental work; reviewed and edited the manuscript.
Abbreviations
AAS: atomic absorption spectrophotometry
C: concentration
Co: initial concentration
Ct:: concentration at time t
D: diffusion coefficient
E: potential
EDX: energy dispersive X-ray spectroscopy
F: Faraday’s constant
Keq: equilibrium constant
n: number of transferred electrons
oz t.: troy ounce
R: universal gas constant
rpm: rotations per minute
SS: stainless steel
T: temperature
U: ion mobility
Vj: migration velocity of ion j
Z: ion charge
Symbols definitions
ΔGo: standard change in Gibbs free energy
δδ: boundary layer thickness