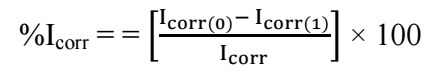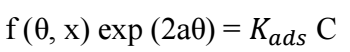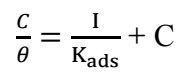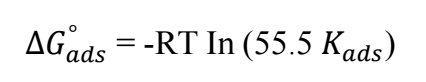Introduction
MS selection for diverse industrial applications is due to its excellent mechanical properties, affordability and high availability 1-2. Steel containers can be used to handle acid, salted and alkali solutions. KOH, Na₂CO₃, NaCl, NaOH and Ca(OH)₂, are some of the corrosive chemicals utilized in industries. Corrosion is a natural phenomenon that is primarily observed in industrial settings, due to its effect on metallic structures and chemical components. Industry risks equipment failure if corrosion-contaminated products are not handled (3) . In terms of product leakage, rehabilitation, replacement and environmental contamination, corrosion has financial repercussions 4. Therefore, controlling corrosion is essential in terms of ecology, economy and practice 5. Prior to plating, painting or storing, chemicals are typically sprayed to metal surfaces, as part of the final finishing steps 6. Scales, dirt and minor rust can be removed from metal surfaces using chemicals 7. Inhibitors are frequently added to process fluids in many industrial activities to reduce metal CR. CI are chemicals that, when applied to corrosive media, at very low concentrations, reduce metals reactivity with their surroundings. By adhering to the metal surface, an inhibitor can lower CR. CI have been the subject of numerous scientific studies 10. Nevertheless, most of what is understood has been found through trial and error research in labs and on the field. There are insufficient principles, formulas or hypotheses to direct the creation or application of CI 11.
Although most inorganic inhibitors have good corrosion-inhibiting properties, they are dangerous, as they can permanently harm human's organs such as kidneys or liver, or can interfere with the body's enzyme system. Therefore, plants are increasingly viewed as sources of green CI, due to environmental considerations. They replace hazardous chemicals in shielding metals and alloys from hostile conditions 8-9. The effective use of plant extracts as corrosion protection for metals in a variety of acidic environments has received some academic attention 12. They have been studied with the aim to modify corrosive conditions where metals are in service. Existing green CI contains Pc such as flavonoids, saponins and tannins. These organic compounds are designed to reduce and prevent corrosion by adhering to the steel surface. Additionally, plants are unquestionably accessible, affordable, and constitute renewable resources 13-14. The main outcomes obtained with the use of plant extracts as inhibitors for MS protection in different alkaline environments are summarized in Table 1.
Table 1: List of plant sources used in investigations of CI on MS in non-acidic media.
| Extract | Corrosive medium | Maximum IE(%) | Adsorption isotherm | Ref. |
|---|---|---|---|---|
| Allium sativum | NaCl | 92 | - | [11] |
| Pentaclethra macrophylla bentham | KOH | 84.02 | Langmuir´s | [15] |
| Psidium guajava | NaOH | 89.0 | Langmuir´s | [16] |
| Beta vulgaris | Simul. oil well water | 94 | - | [17] |
| Agri-food wastes | NaCl | 98.0 | - | [18] |
| Thymus vulgar | NaCl | 80.49 | Temkin | [19] |
| Arecanut husk | NaOH/HCl | 94. 347 | Langmuir´s | [20] |
| Pterocarpus soyauxii taub | Na2CO3 | 70.05 | Langmuir´s | [21] |
| Azadirachta indica | Ca(OH)2 + NaCl | 86 | - | [22] |
| Olea europaea | NaOH + NaCl | 91.9 | - | [23] |
| Seaweed | Saline form. water | 93.64 | Temkin’s | [24] |
25-26 have successfully used CO plant stems as CI on MS in H2SO4, and the results demonstrated their feasibility and efficacy in inhibiting corrosion in selected environments, with high IE(%) of 93 and 94.34%, respectively. However, despite the impressive IE(%) of CO stems, there has been no investigation on their leaves for the same purpose, in an alkaline environment. Therefore, this paper examines COLE effectiveness in mitigating MS corrosion in KOH. This will be the first report on the application of this plant extract in an alkaline medium. Pc study and techniques, such as PDP and FTIR, were used to characterize MS, and to test IE(%) of COLE.
Experimental work
Materials
MS composition was 0.15% C, 0.36% P, 0.03% Si, 0.6% Mn, and the remainder Fe. MS coupons were cut into dimensions of 2 × 2 × 0.2 cm, with a 0.05 cm hole drilled in the center. They were degreased and polished with emery paper, to expose them for corrosion attacks.
COLE procurement and preparation
Fresh CO leaves were bought from Abakaliki international market, Nigeria. They were dried for six days, under hot sun, to remove moisture content. Thereafter, they were ground to fine powder and filtered, to obtained fine particles. The obtained raw fine powder was then stored in a container. The resulting filtrate was utilized as CI in its purest form. Ct of 1, 1.5, 2, 2.5 and 3 g/L COLE were used in 1.5 M KOH test solutions.
Qualitative analysis of Pc
By looking for secondary metabolites, such as steroids, saponins, alkaloids, flavonoids, tannins, phenols and terpenes, qualitative analysis was done to determine the presence of these Pc on the powdered CO leaves, according to the method of 27.
PDP test
PDP method was also carried out in accordance with instructions given in 28, where a 3-electrode cell was employed for the analysis, with SCE, graphite and MS coupon as reference, counter and working electrodes, respectively. Table 3 shows the results.
FTIR analysis
FTIR spectrophotometer was employed to perform analyses on MS coupons immersed in KOH without and with COLE, to observe its residue absorbed onto the alloy surfaces.
Results and discussion
Pc analysis of COLE
Results for Pc analysis of COLE are shown in Table 2.
Table 2: Pc analysis of COLE.
| Pc | Results |
|---|---|
| Steroids | ++ |
| Saponins | ++ |
| Flavonoids | ++ |
| Tannins | ++ |
| Alkaloids | + |
| Phenols | + |
| Terpenes | ++ |
Fairly present +, heavily present ++
CI calculation using PDP method
Icorr(%) determination was done by PDP (Fig. 1).
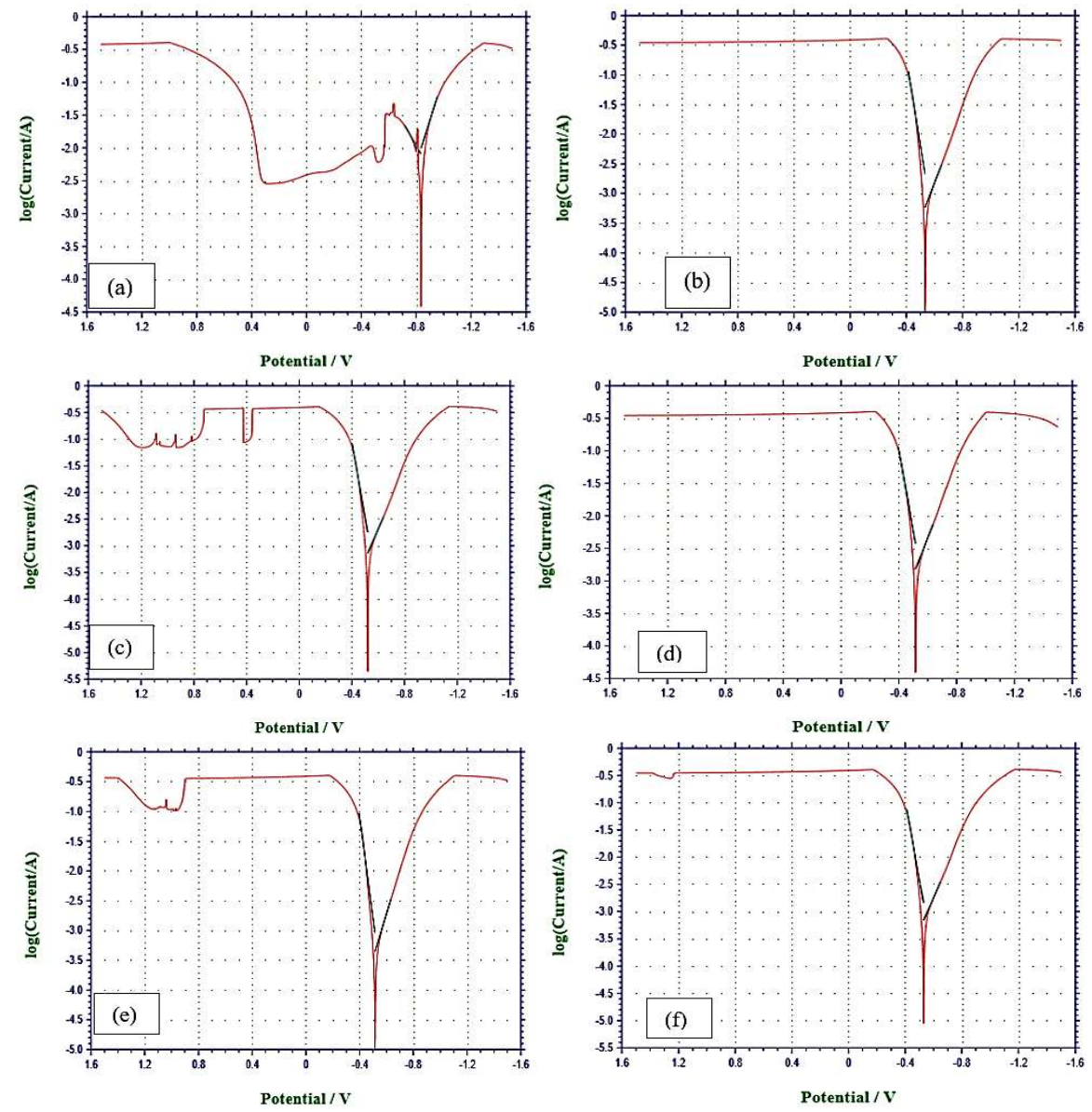
Figure 1: PDP behavior of MS in- (a) blank 1.5 M KOH; and with COLE in the Ct of - (b) 1; (c) 1.5; (d) 2; (e) 2.5; and (f) 3 g/L.
Calculations were carried out using eq.(1) 29.
where Icorr(0) and Icorr(1) are Icorr without and with inhibitor, respectively. Corrosion parameters collected from PDP, as listed on Table 3, were plotted (Figs. 2 and 3).
Table 3: PDP corrosion parameters obtained in 1.5 M KOH without and with COLE, at 298 K.
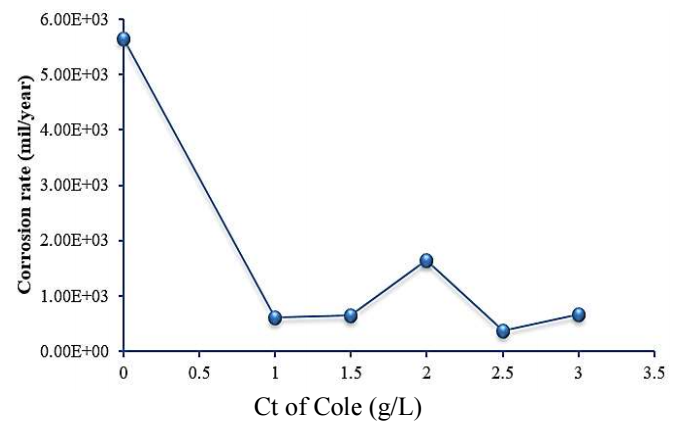
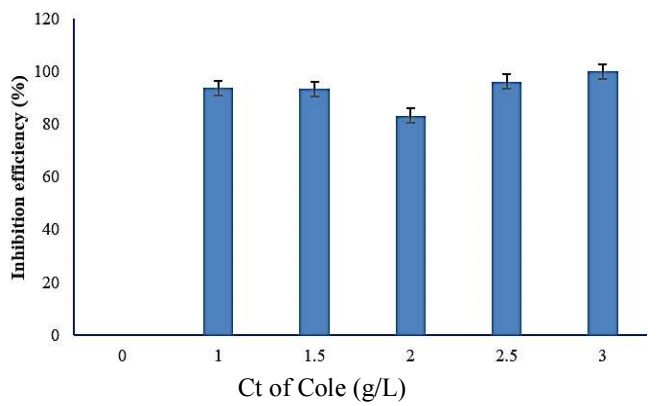
| Ct of COLE (g/L) | Ecorr (V) | Icorr (A) | CR (mm/year) | IE(%) | SC (θ) |
|---|---|---|---|---|---|
| 0 | -0.834 | 1.227e-002 | 5.644e+003 | ||
| 1 | -0.532 | 7.665e-004 | 6.162e+002 | 93.75 | 0.9375 |
| 1.5 | -0.519 | 8.171e-004 | 6.568e+002 | 93.34 | 0.9334 |
| 2 | -0.515 | 2.060e-003 | 1.656e+003 | 83.21 | 0.8321 |
| 2.5 | -0.514 | 4.725e-004 | 3.799e+002 | 96.14 | 0.9614 |
| 3 | -0.529 | 8.321e-004 | 6.689e+002 | 99.93 | 0.9993 |
Fig. 2 shows that, generally, CR decreased with higher Ct of COLE. This decrease in CR can be linked to the physical adsorption process, which took place as COLE molecules were absorbed onto the MS surface 30. CR was stable with 1 and 1.5 g/L COLE, but increased with 2 g/L, and decreased with 2.5 g/L. Lowest CR was obtained with 2.5 g/L COLE.
Fig. 3 displays IE(%) of COLE, which decreased with Ct from 1 to 2 g/L, and increased with 3 g/L, to reach its highest value of 99.93%.
FTIR
Fig. 4 shows FTIR remarkable broad peaks at stretching vibrations of 3859.7, 3833.6, 3814.9 and 3844.7 cm-1, for coupons A to D, respectively, with COLE.
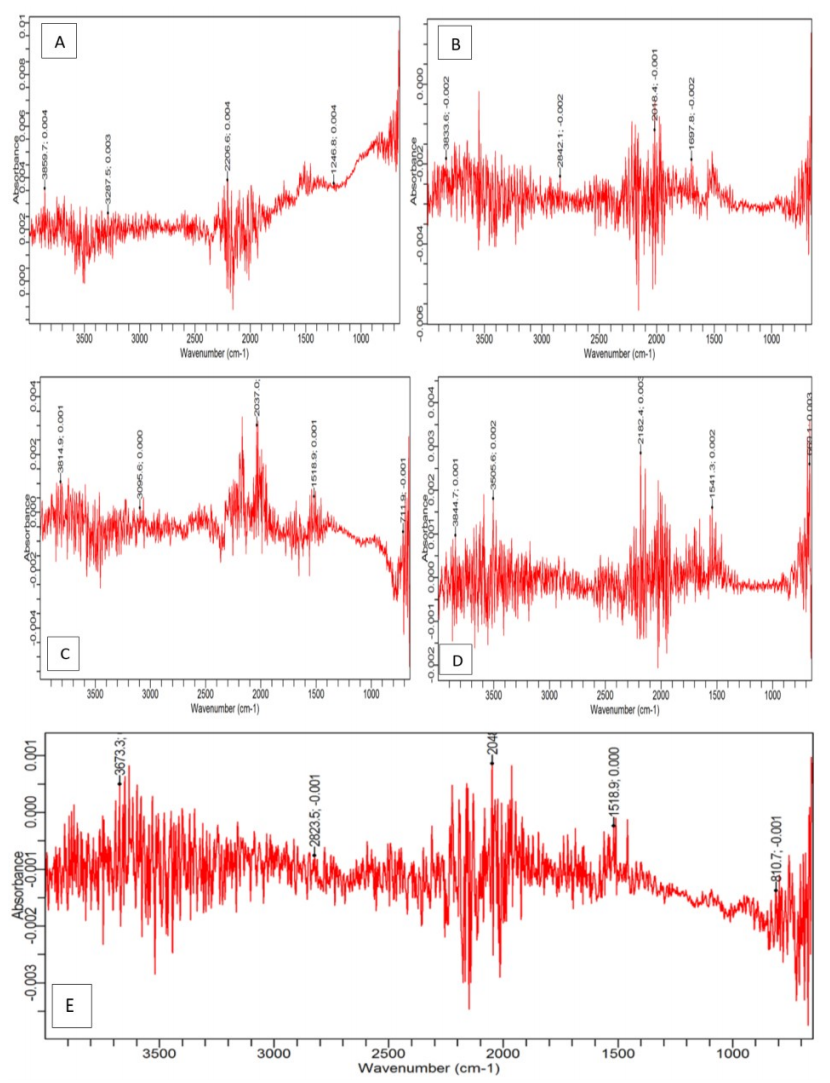
Figure 4: FTIR spectra for MS in 1.5 M KOH with COLE at Ct of- (A) 1; (B) 1.5; (C) 2; (D) 2.5; and (E) 0 g/L (control).
A lesser broad peak of 3673.3 cm-1 was found on the control (E). This stretching vibration could be due to the presence of N-H and/or O-H bonds. 2206.6, 2842.1, 2037.0, 2182.4 and 2823.5 cm-1 peaks were stretching vibrations caused by C-H and C=C bond groups. 1246.8, 1697.8, 1518.9, 1541.3 and 1518.9 cm-1 peaks were recognized as stretching vibrations linked to C-H vibration of CH3 group 31. Any other stretching vibrations below 1000 cm-1 were due to C-H vibration of the aromatic and/or aliphatic group, all in agreement with literature (31).
Adsorption isotherm
Generally, adsorption isotherms are crucial and helpful in determining and/or understanding the nature of CI mechanism, and the interaction between extract molecules and the MS surface 32. The most consistently utilized isotherms are Frumkin’s, Freundlich’s, Langmuir’s, Temkin’s and Flory-Huggins’, of which mechanisms are explained by the general formula shown in eq. (2) 24:
where f (θ, x) is the configurational factor that solely depends upon the physical adsorption model and the assumptions underlying the isotherm derivation, a is the interaction factor, θ is SC (IE/100) and C is Ct of COLE in 1.5 M KOH.
The results of this polarization investigation were found to fit Langmuir’s adsorption isotherm, according to eq. (3) 33:
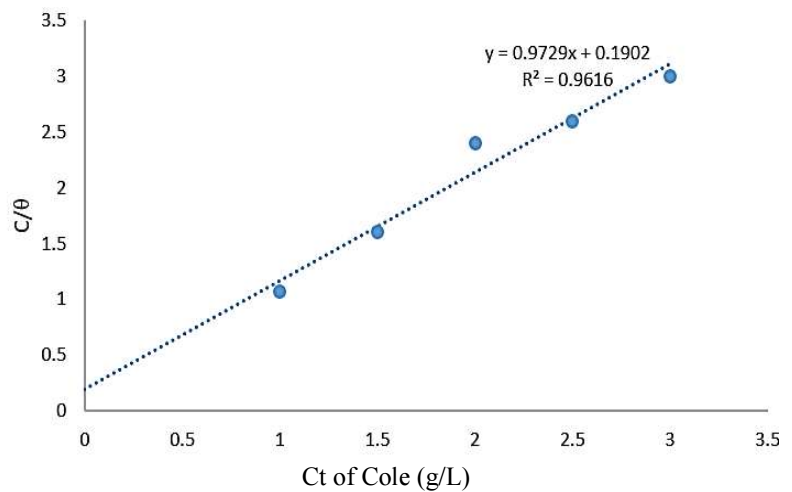
The plot of C/θ against C, at 298 K, gave a straight line, as seen in Fig. 5.
Kads was determined to be 0.9729 g/L, and R2 was found to be 0.9616, which is very close to unity. It was said that Kads value could be associated with the strength of adsorption forces between inhibitor molecules and metal surface 33. As the linear plot had high R2, this could show that inhibition was due to COLE active organic compounds adsorbion onto the MS surface, which obeyed Langmuir’s isotherm. Also, the determined positive value (0.1902) of the molecular interaction parameter known as “a” shows that there were attraction forces in the adsorption layer on the MS surface. This is in agreement with 24.
Kads was related to 〖∆𝐺〗_𝑎𝑑𝑠^° , by eq. (4) 14:
where 55.5 is molar Ct of water in the bulk solution, R is gas constant and T is temperature.
〖∆𝐺〗_𝑎𝑑𝑠^° value calculated at 298 K was -9.882 kJ/mol. Its negative value indicates the possibility of the inhibitor spontaneous nature adsorption process. 〖∆𝐺〗_𝑎𝑑𝑠^° value of -20 kJ/mol or any other less negative values confirm physisorption, while those lower than -40 kJ/mol are taken to be chemical adsorption. Therefore, COLE adsorption onto the MS surface followed physical adsorption mechanism, in agreement with 34.
Conclusion and future outlook
This research successfully investigated COLE as a good CI. It was concluded that COLE proved to be a good eco-freiendly organic inhibitor capable of controlling and preventing CR of MS. COLE had very high IE(%) values of 96.14 and 99.93%, with Ct of 2.5 and 3 g/L, respectively.
Ecorr decreased from -0.824 to -0.529 V, while Icorr reduced from 1.227e- 002 to 8.321e-004 A, and there was a maximum SC of 0.9993. These results proved that COLE was able to control MS corrosion in a KOH solution.
Thermodynamic and other adsorption parameters revealed that COLE interaction with the MS surface was through physical adsorption, obeyeing Langmuir’s isotherm.
This research contributed to understand MS corrosion in an alkaline solution, which was inhibited by COLE.
Further studies employing COLE as CI for MS and other metals, in various alkaline environments, are suggested, so as to compare them with the high IE(%) values recorded in this work.
Acknowledgement
The authors of this research paper are appreciative of the support and encouragement provided by their colleagues in the departments of Materials and Metallurgical Engineering at Nnamdi Azikiwe University in Awka, Anambra State, Nigeria, and in the Chemistry Laboratory at Alex Ekwueme Federal University Ndufu-Alike in Ikwo, Ebonyi State, Nigeria.
Authors’ contributions
Chukwuka Ikechukwu Nwoye: offered intellectual assistance with data collection, analysis and interpretation; proofread the work, made the required modifications and approvrf the final version to be published; primarily contributed to the design and technical direction. Uzoma Samuel Nwigwe: conducted the study; analyzed the data and interpreted the results; wrote the article as the primary author, and read it; made a moderate contribution to the analysis, conceptualization and design.
Abbreviations
Ca(OH)₂: calcium hydroxide
CI: corrosion inhibitor/inhibition
COLE: Corchorus olitorius leaves extract
CR: corrosion rate
Ct: concentration
Ecorr: corrosion potential
FTIR: Fourier-transform infrared spectroscopy
H2SO4: sulphuric acid
HCl: hydrochloric acid
Icorr: corrosion current
IE(%): percentage inhibition efficiency
KOH: potassium hydroxide
MS: mild steel
Na₂CO₃: sodium carbonate
Na2SO3: sodium sulfite
NaCl: sodium chloride
NaOH: sodium hydroxide
Pc: phytochemical
PDP: potentiodynamic polarization test
R2: coefficient of determination
SC: surface coverage
SCE: saturated calomel electrode