Introduction
Portugal’s first case of coronavirus disease 2019 (COVID- 19) was notified on March 2nd, 2020. Since then, over 800 000 cases were diagnosed and 16 000 deaths were reported.
Knowledge about the clinical characteristics and outcomes of COVID-19 patients is persistently increasing due to a growing body of evidence from international reports, mainly from China1,2and United States of America (USA),3 but also from some European centres.4-6 Demographic, environmental, cultural characteristics and comorbidities prevalence likely varies between countries. Therefore, the current knowledge may not necessarily be illustrative of the Portuguese reality. In the other hand, our data could contribute to a global analysis regarding this disease.
Our aim is to describe the demographic characteristics, clinical course, management and outcomes of hospitalized patients with COVID-19 in a Portuguese tertiary center. As the pandemic is quickly evolving, we also sought to investigate potential risk factors for in-hospital mortality to further allow the development of prognostic models and tailor the daily clinical management of the individual patient.
Material and Methods
STUDY DESIGN AND PATIENTS
This ambispective cohort study included all COVID-19 inpatients aged 18 years-old or older, admitted to Hospital de Santa Maria (Lisbon, Portugal). Patients were recruited from March 3rd to August 3rd, 2020, first outbreak. Patients entered the study retrospectively and prospectively before and after 27th of April. Patients were followed until they were discharged, lost to follow-up or died. All patients had a positive test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by reverse transcriptase polymerase chain reaction. We have excluded asymptomatic patients, pregnant women, patients that were admitted due to another condition and all those who were hospitalized due to social/sanitary reasons (e.g., the inability for social isolation).
Readmissions were also excluded (4 patients with more than 1 admission during this period). The clinical outcomes were recorded until September 3rd, 2020, corresponding to the day the last patient reached the primary outcome. The study was approved by the Hospital de Santa Maria’s ethics committee (No 178/20) in accordance with the Helsinki Declaration statements.
The requirement for the written informed consent was waived.
DATA COLLECTION
Epidemiological, demographic and clinical data were obtained through clinical interviews and from electronic medical records. Data was collected using standardized data collection form and stored according to applicable legislation. Comorbidities and clinical data were either self-reported by the patients and/or extracted from all existing medical records.
Laboratory and inpatient treatment modalities were assessed through patient electronic files and prescription software. The anonymity of patients was warranted.
DEFINITIONS AND OUTCOME
Presentation symptoms were considered until the third day of hospitalization. Fever was defined as tympanic temperature of at least 38.0oC. Time of the first symptoms noticed was considered disease onset. Imaging and laboratory results corresponded to the first available result from admission until the third day of hospitalization. Patients were classified based on their performance status using the Clinical Frailty Scale (CFS)7as follows: autonomous (CFS 1-4), partially dependent (CFS 5-6) and totally dependent (CFS 7-9). Acute kidney injury was diagnosed according to Acute Kidney Injury Network criteria.8 Acute respiratory distress syndrome (ARDS) was diagnosed according to Berlin criteria.9 Acute lung injury (ALI) was diagnosed according to the American-European Consensus Conference on ARDS.10 Cancer included any type of active solid and/or hematological cancer under active surveillance and/or treatment.
Cardiovascular disease (CVD) included all the following: aortic aneurysm disease, cardiomyopathy of any cause, coronary artery disease, heart failure of any cause, heart valve disease, peripheral artery disease and pulmonary hypertension. Chronic kidney disease (CKD) was diagnosed according to the “Kidney Disease: Improving Global Outcomes (KDIGO)”.11 Obesity was defined as body mass index (BMI) equal or higher than 30 kg/m2. Radiological SARS-CoV-2 infection suggestive findings were as follows: chest radiography - unilateral or bilateral patchy pulmonary infiltrates and interstitial abnormalities; Chest computerized tomography (CT) scan - unilateral or bilateral pulmonary ground-glass abnormalities, honeycomb findings, organizing pneumonia. Clinical discharge was defined by concomitant absence of fever and oxygen requirements for at least 2 consecutive days. The outcome date was defined as the day of the patients’ death or the hospital discharge date, due to clinical criteria. The total length of hospital stay was determined from the date of admission until the outcome date. The primary outcome was in-hospital all-cause mortality.
Illness severity was accessed according to development of ARDS/ALI, intensive care unit (ICU) admission and invasive mechanical ventilation (IMV) need.
STATISTICAL APPROACH
We used STATAR (version 16) and SPSSR software (version 26.0) for statistical analysis. Continuous and categorical variables were presented as median ± interquartile range (IQR) and number (%), respectively. We used the Mann-Whitney U test, χ2 test or Fisher’s exact test to compare differences between groups when appropriate. The minimum and maximum values will be shown whenever pertinent. We used univariable and multivariable logistic regression models to explore risk factors associated with the primary outcome.
Variables were eligible for multivariable analysis if their between- group differences were significant. Patients were suppressed if they were lost to follow-up or reached the primary outcome. We considered a two-sided α of less than 0.05 as statistically significant.
Results
DEMOGRAPHICS, COMORBID CONDITIONS AND CLINICAL MANIFESTATIONS
Four hundred and thirty-two patients were admitted during the study period. After applying the exclusion criteria, 279 were eligible for further analysis (Fig. 1).
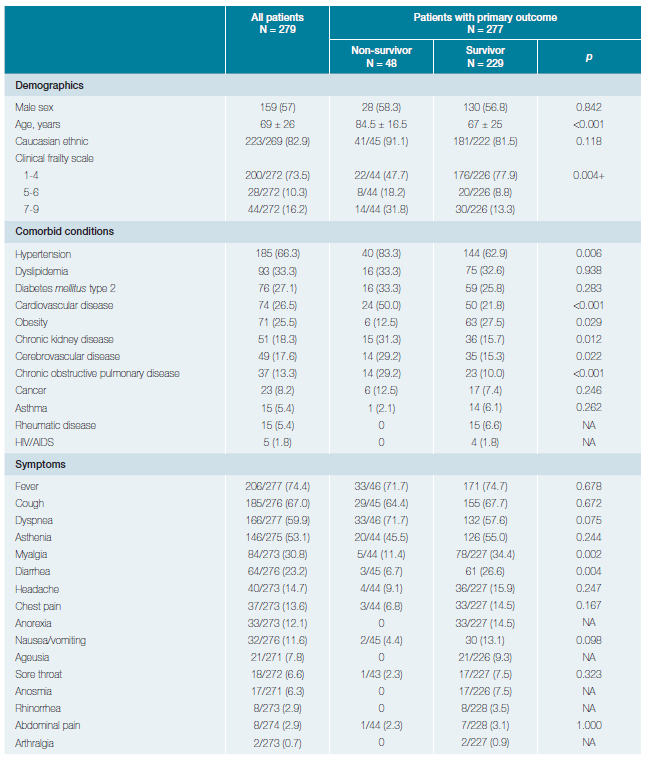
The baseline characteristics of our cohort are shown in Table 1. Fifty-seven percent of the patients (n = 159) were male, and the vast majority were of Caucasian ethnic origin (82.9%).
The median age was 69.0 ± 26.0 years. Most patients were independent (73.5%), followed by totally dependent (16.2%) and partially dependent (10.3%) upon hospital admission. High blood pressure (HBP) was the most prevalent comorbid condition (66.3%), followed by dyslipidemia (33.3%), diabetes mellitus type 2 (27.1%), cardiovascular disease (CVD) (26.5%) and obesity (25.5%). Nearly three-fourths of the patients (74.4%) had fever before or during the first three days of hospitalization. Cough was reported in 67.0% of patients, followed by dyspnea (59.9%), asthenia/fatigue (53.1%) and generalized myalgia (30.8%). The least frequent symptoms were, by descending order, anosmia (6.3%), rhinorrhea (2.9%), abdominal pain (2.9%) and arthralgia (0.7%). The median time from disease onset to hospital admission was 5.0 } 4.0 days, ranging from 0 to 21 days (data not shown).
Table 1: Baseline characteristics description of all patients and comparison according to primary outcome of patients with COVID-19.
Data is shown as number (%) for categorical variables and median ± interquartile range for continuous variables. The denominators of patients who were included in the analysis are provided if it differed from the overall numbers within the group. AIDS, acquired immunodeficiency syndrome; ALI, acute lung injury; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; CRP, c-reactive protein; CT-scan, computerized tomography scan; F, female; ICU, intensive care unit; IL-6, interleukin 6; IMV, invasive mechanical ventilation; M, male +χ2 test comparing all subcategories.
Table 1 (cont.): Baseline characteristics description of all patients and comparison according to primary outcome of patients with COVID-19.

Data is shown as number (%) for categorical variables and median ± interquartile range for continuous variables. The denominators of patients who were included in the analysis are provided if it differed from the overall numbers within the group. AIDS, acquired immunodeficiency syndrome; ALI, acute lung injury; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; CRP, c-reactive protein; CT-scan, computerized tomography scan; F, female; ICU, intensive care unit; IL-6, interleukin 6; IMV, invasive mechanical ventilation; M, male +χ2 test comparing all subcategories.

Data is shown as number (%) for categorical variables and median ± interquartile range for continuous variables. The denominators of patients who were included in the analysis are provided if it differed from the overall numbers within the group. AIDS, acquired immunodeficiency syndrome; ALI, acute lung injury; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; CRP, c-reactive protein; CT-scan, computerized tomography scan; F, female; ICU, intensive care unit; IL-6, interleukin 6; IMV, invasive mechanical ventilation; M, male +χ2 test comparing all subcategories.
RADIOLOGIC AND LABORATORY FINDINGS
Of the 272 chest radiographies and 150 chest CT-scans that were performed at the time of admission, 76.1% and 88.7% revealed suggestive findings of SARS-CoV-2 infection, respectively. On admission, lymphopenia (lymphocyte count <1.0x109/L) was the most frequent blood count abnormality (45.7%). Severe lymphopenia (lymphocyte count ≤0.5x109/L) was found in 8.3% of patients. D-dimer levels were higher than 0.25 μg/mL in 73.9% of patients, but only 21.0% presented values equal or higher than 1 μg/mL. Most patients showed increased inflammatory markers: elevated ferritin levels in 86.3%, C-reactive protein (CRP) in 74.8% and interleukin (IL) 6 in 60.5% of the patients.
INPATIENT TREATMENT, CLINICAL COURSE AND OUTCOMES
Antiviral therapy was the most frequently administered treatment (71.5%), followed by hydroxychloroquine (52.8%), steroids (29.0%) and tocilizumab (5.5%). Antibiotic therapy was used in 49.5% of the patients. The time from disease onset and hospital admission to outcome was 17 ± 12 days and 10 ± 13 days respectively. During the study period, two patients were lost to follow-up and 48 patients (48/277, 17.3%) died. Approximately 32%, 31.9% and 20.8% of patients developed ARDS/ALI, were admitted in the ICU and needed IMV, respectively. The timeframe for each secondary outcome is displayed in Table 1.
MORTALITY AND SEVERE DISEASE DETERMINANTS
Patients who died were older (84.5 ± 16.5 vs 67.0 ± 25.0 years, p < 0.001) and had higher proportion of high CFS (CFS ≥5, 50.0% vs 22.1%, p = 0.004). Gender (male, 56.8% vs. 58.3%, p = 0.842) and the frequency of Caucasian ethnicity (81.5% vs 91.1%, p = 0.118) were equally distributed between survivors and non-survivors. Time from symptom onset to hospital admission (3.0 ± 4.0 vs 6.0 ± 4.0 days, p = 0.008), symptom onset to outcome (12.0 ± 22.0 vs 17.0 ± 11.0 days, p = 0.025), but not from hospital admission to outcome (7.5 ± 16.0 vs 11.0 ± 11.0 days, p = 0.120), were significantly shorter in the non-survivor compared to survivor group.
HBP (83.3% vs 62.9%, p = 0.006), CVD (50.0% vs 21.8%, p < 0.001), CKD (31.3% vs 15.7%, p = 0.012) and chronic obstructive pulmonary disease (COPD) (29.2% vs 10.0%, p < 0.001) were significantly more prevalent in COVID-19 patients who died compared to those who survived. Obesity was the only comorbid condition that was significantly more prevalent in the survivor group (27.5% vs 12.5%, p = .029). Symptoms were similarly reported by survivors and non-survivors, except for generalized myalgia (34.4% vs 11.4%, p = 0.002) and diarrhea (26.6% vs 6.7%, p = 0.004) that were significantly more prevalent in the survivors compared to non-survivors. Patients who died had higher proportion of anemia (45.8% vs 29.8%, p = 0.032), leukocytosis (33.3% vs 10.1%, p < 0.001), severe lymphopenia (20.8% vs 5.7%, p = 0.001) and d-dimer levels equal or higher than 1 μg/mL (50.0% vs 15.8%, p < 0.001).
Regarding the inflammatory markers, only the median plasma level of CRP (13.0 ± 16.2 vs 9.1 ± 11.8 mg/dL, p = 0.025) and IL-6 levels (145.4 ± 95.7 vs 48.0 ± 47.8 pg/mL, p = 0.004), but not ferritin levels (564.5 ± 2231.0 vs 841.0 ± 1103.0 ng/mL, p = 0.578), differed significantly between these two groups.
Finally, in-patient treatments were similarly distributed between all patients. Only the proportion of antibiotic administration differed between the non-survivors and survivors (69.8% vs 45.2%, p = 0.003).
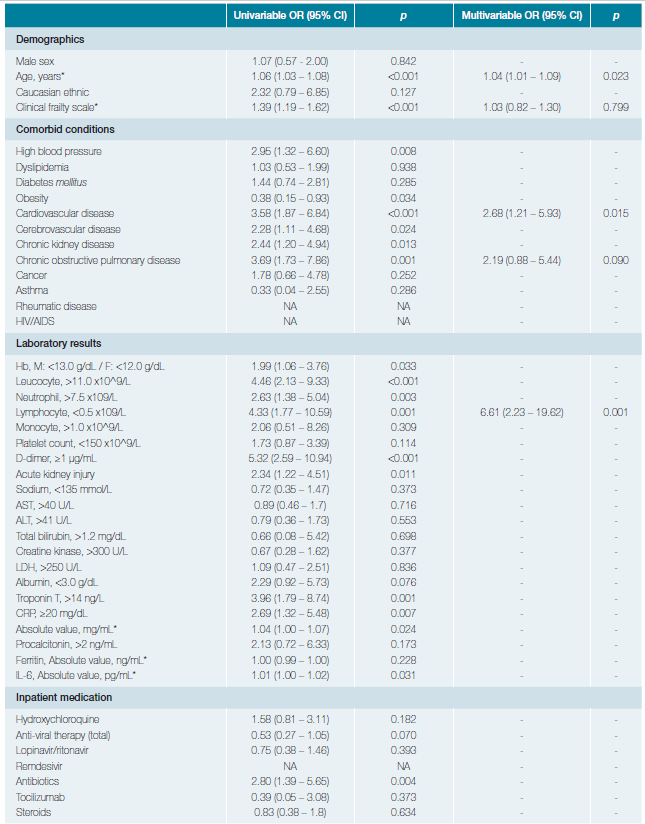
Table 2 displays the risk factors associated with in-hospital mortality.
In univariable analysis, the odds of in-hospital death were higher in patients with HBP (OR 2.95, 95% CI 1.32 - 6.60, p = 0.008), CVD (OR 3.58, 95% CI 1.87 - 6.84, p < 0.001), cerebrovascular disease (OR 2.28, 95% CI 1.11 - 4.68, p = 0.024),
CKD (OR2.44, 95% CI 1.20 - 4.94, p = 0.013) and COPD (OR 3.69, 95% CI 1.73 - 7.86, p = 0.001). Conversely, obesity was not associated with the odds of death (OR 0.38, 95% CI 0.15 - 0.93). Age (per-unit increase OR 1.06, 95% CI 1.03 - 1.08, p < 0.001), anaemia (OR 1.99, 95% CI 1.06 - 3.76, p = 0.033), white blood cell count >11x109/L (OR 4.46, 95% CI 2.13 - 9.33, p < 0.001), neutrophil count >7.5x109/L (OR 2.63, 95% CI 1.38 - 5.04, p = 0.003), lymphocyte count ≤0.5x109/L (OR 4.33, 95% CI 1.77 - 10.59, p = 0.001), d-dimer ≥1μg/mL (OR 5.32, 95% CI 2.59 - 10.94, p < 0.001), CRP (per-unit increase OR 1.04, 95% CI 1.00 - 1.07, p = 0.024) and IL-6 (per-unit increase OR 1.01, 95% CI 1.00 - 1.02, p = 0.031) levels at admission were also significantly associated with death.
In multivariable analysis, we found that age, CVD and severe lymphopenia maintained their significant association with the odds of death. Noteworthy, after several multivariable arrangements, the use of antibiotics was not associated with the odds of dying (data not shown).
Discussion
To the best of our knowledge, this is one of the first studies to analyze a cohort of adult inpatients with COVID-19 in Portugal and to identify several risk factors for in-hospital death in this population. We believe that this data brings insightful information about hospitalized patients with COVID-19 from the first pandemic outbreak in our country.
We report a similar prevalence of the most frequent comorbidities, namely HBP, dyslipidemia, diabetes, CVD and obesity, regarding most European studies.4,5,12,13The high prevalence of these cardiovascular diseases is systematically reported and frequently associated with adverse outcomes in COVID-19 patients.1,3-5,12,14Patients with such diseases, usually older, tend to decompensate their chronic illnesses and seek for medical care. The overrepresented proportion of some of these comorbid conditions may, therefore, arise from a possible selection bias in most studies. We also observed that HBP, CKD, COPD and especially CVD were associated with an increase in the risk of death in our cohort. Despite the recognized negative impact of obesity in the clinical course of COVID-19 patients,15we were surprised to find that obesity was associated with lower risk for death in our patients (OR 0.38, 95% CI 0.15 - 0.93, p = 0.034). Even though we report a similar prevalence of obesity as our last national enquiry,16 we believe we might lack sensitivity compared to others who have reported obesity in categories13 and even as continuous values of BMI.17
In our cohort, the most frequent symptoms upon admission were fever, cough, dyspnea and asthenia, which were similar to those reported in other studies.1-5,12,14Unlike us, some authors have described those symptoms more frequently among non-survivors.9 On the other hand, we report nausea, anosmia, ageusia and generalized myalgia less frequently. These symptoms are associated with milder forms of disease, usually in patients that do not require hospitalization.6 The median time from disease onset to hospital admission was in accordance with published data.18 Similarly to others, we have noticed that deceased patients are admitted at an earlier stage of the disease and have a shorter hospital stay, suggesting a more severe initial presentation and faster deterioration.1,17
The positive relationship between a higher mortality and some laboratory markers, such as increased CRP, IL-6 and d-dimer, has already been reported1 and might reflect the hyperinflammatory state known to occur in severe SARS-CoV-2 infections.19 Moreover, our results show that not only lymphopenia was associated with mortality, but also the increase in the total leukocyte count and, more importantly, neutrophil count, were also associated with death. We did not calculate the neutrophil-to-lymphocyte ratio, which has been increasingly recognized as an important predictor of severity and mortality in other studies.20,21However, it remains difficult to interpret it as simply a sign of a superimposed bacterial infection22,23or a surrogate of a dysregulated immune response,21 by which COVID-19 is known for.19,21,24
Treatment options were empirical and experimental based.
In our institution, the inpatient treatment regimens were in line with other studies1,2,4,5,25and international recommendations at that time. It is now known that no benefit was observed with lopinavir-ritonavir treatment when compared to supportive care in hospitalized adult patients with severe COVID-19.26 Therefore, we were not surprised to observe that these antiviral therapies did not change the risk of mortality in our study. The same is also true for remdesivir that had little or no effect on hospitalized patients with COVID-19 according to recent clinical trials.27 In our sample, we cannot comment on the effectiveness of remdesivir due to the small number of cases treated with remdesivir.
Frailty scores are frequently used in clinical decision-making, yet they are scarcely reported in most COVID-19 series. Despite their age, nearly three-quarters of our patients were considered fit or vulnerable, but not frail, according to the CFS, and this difference was even more pronounced when we compared survivors with non-survivors (CFS 1-4, 77.9% vs 47.7%, p = 0.004). The impact of CFS on disease outcomes was even more significant (p < 0.001) in a logistic model, further supporting its’ applicability in clinical practice as has been stated by others.28
Our cohort was composed predominantly by Caucasian males which is in conformity with the majority of published European series demographic characteristics. Age is one of the most important risk factors for mortality. Accordingly, we also found a significant relationship between age and death, with an increase of 60% in the chance of dying for every 10 years increase in age (Table 2). The median age of our patients was 69 years, which is higher compared to data from China2 (47 years) and from the USA3 (63 years). This disparity between median ages is probably related to our inclusion/exclusion criteria and to the fact that the Portuguese population is remarkably older when compared to other European and non-European countries.
In our series, the in-hospital mortality rate was 17.3%. Even though population-based studies13 tend to have lower mortality rates than hospital-centered ones, and despite our rigorous patient selection criteria, we still report a lower mortality rate than other in-patient series, like New York’s (21%),3 Wuhan’s (28.3%),1 the United Kingdom’s (37%)12 and Italy’s (43.6%).5
In a large multicenter retrospective study from Spain, Casas-Rojo JM et al4 showed that 21% have died and only 8.3% were admitted to the ICU. The ARDS prevalence was similar to ours (32.2% vs 33.1%). Even though our results are not as representative as theirs when it comes to cohort size, we consider that these differences with regard to mortality, but also to other severity outcomes, deserves a special attention, as we share many demographic characteristics, living conditions and access to health care facilities with the Spanish population.
These differences could be explained by the different timing and magnitude of the pandemic and its pressure on the healthcare system, which was earlier and more devastating compared to Portugal, giving us an advantage in applying timely confinement measures during this first outbreak. Although the Portuguese’s national health system has the lowest ratio of number of ICU beds per 100 000 inhabitants (4.2) and one of the lowest number of ventilators (1400) in the European Union, our hospitals were never on the verge of rupture during this first wave of the pandemic, compared to Spain, France and Italy.
Our study has several limitations. First, despite our active role as clinicians, we were not able to have a direct interaction with all patients, neither did we have control over which laboratory or imaging exams were performed, hence some data are missing. Secondly, our restricted sample size limits the interpretation of our findings and may not be representative of our total national data. We also have some strengths. As all our patients have finished the study, our mortality rate is accurate. Furthermore, the ambispective nature of the study warrants some preciseness in data collection. Finally, we highlight the role of fragility as an important risk factor to be taken into account.
Conclusion
In conclusion, the present work represents one of the first studies describing and analyzing the demographic, clinical characteristics and outcomes of hospitalized patients with COVID-19 in Portugal. We found similar clinical presentation
symptoms and comorbid conditions as those reported internationally, some of them clearly linked with an increased risk of death, namely CVD and COPD. In addition to age, the potential use of a frailty scale as a prognostic factor should help clinicians to identify patients with poor prognosis at an early stage. We report a lower mortality rate compared to studies in hospitals in other countries during the first outbreak.