Introduction
Upper extremity deep venous thrombosis (UEDVT) repre-sents about 4% to 10% of all deep venous thrombosis (DVT) cases, but its incidence is increasing due to higher frequency of intravenous catheter use.1,2The axillary and subclavian veins are the most common site of thrombosis, but the more distal brachial, ulnar, and radial veins may also be involved.2
Based on pathogenesis, UEDVT is classified as primary or secondary. Up to one third of all are primary thrombosis, further classified as idiopathic, if no predisposing anatomical or haematological factors are identified, or effort thrombosis when associated with strenuous activity.3 The secondary UEDVT ac-counts for most cases and is mainly associated with malignancy and implantable devices such as catheters, prosthetic, or cardiac material.2,3
Also referred to as Paget-von Schroetter syndrome (PSS), effort thrombosis is a rare condition, first described by James Paget in 1875 and von Schroetter in 1884, and later designated as the eponym in 1949 by Hughes.4 With an incidence of around 1-2 per 100 000 patients per year, PSS accounts for 1%-4% of all venous thrombosis episodes, affecting mainly young men, with an average age of 30 years and a male-to-female ratio of 2:1.5
This syndrome is classically reported in individuals who per-form repetitive upper arm movements, such as athletes (gymnasts, swimmers, tennis or baseball players, weightlifters) and certain workers (mechanics, painters, electricians).4,5Heavy exertion is thought to cause venous microtrauma, which in turn leads to activation of the coagulation cascade, leading to significant thrombosis, particularly if mechanical compression of the vessel is also present.6
Clinical features are broad, non-specific, and its severity is generally proportional to the degree of venous obstruction. Typical symptoms include unilateral upper extremity discomfort, oedema, and fatigue.2 If the more proximal superior vena cava is involved, cervical and/or facial swelling, cyanosis, headache, blurred vision, cough, dyspnoea, and orthopnoea may be noted. Distended cervical veins and/or prominent superficial collateral veins may appear on the shoulder and anterior chest wall (Urschel´s sign).7 Most patients will have symptoms within 24 hours of the inciting event, but not infrequently PSS may be asymptomatic.4
We report an unusual case of primary left axillo-subclavian venous thrombosis in a middle-aged man with a recent routine of moderate upper limb exercise and no other obvious risk factors for thrombosis.
Case Report
A 50-year-old male patient was admitted to the emergency department with a history of progressive painful oedema in the left upper limb for 2 days. It was not associated with dyspnoea, chest pain, palpitations, haemoptysis, or syncope. There was no history of trauma, insect bite, surgery, prolonged immobiliza-tion, or strenuous activity; however, he mentioned that he used to do 20 dips a day on the stairs of his workplace (he was a wai-ter) in the last few weeks. The patient had no remarkable past medical history except for dyslipidaemia and smoking. He was not taking any medications, notably steroids or supplements.
The physical examination revealed tense oedema, associated with erythema and pain, from the wrist to the left shoulder. There were no superficially engorged veins on the arm or the chest, neither palpable lymphadenopathy. All peripheral pulses were palpable and capillary refill time was <2 seconds. The pa-tient was afebrile, hemodynamically stable, eupnoeic at rest, with oxygen saturation of 97% in room air.
Laboratory studies (Table 1), including blood count, coagulation, and biochemistry, were in the normal range, except for elevated D-Dimers (900 ng/L). Doppler ultrasonography (Fig. 1) of the left upper limb revealed extensive thrombosis of the subclavian (partially occlusive), axillary and brachial veins (totally occlusive). Contrastenhanced computed tomography scan (Fig. 2) confirmed the presence of left axillo-subclavian venous thrombosis, as well as segmental pulmonary embolism (PE) of the right inferior lobar artery; there were no structural abnormalities obstructing the thoracic outlet.
Table 1: Analytic results at baseline
| Parameter | Value | Reference Value |
|---|---|---|
| Hemoglobin (g/dL) | 15.4 | 13.0-17.0 |
| White blood count (x 109/L) | 9.6 | 4.0 - 11.0 |
| Platelet (x 109/L) | 179 | 150 - 450 |
| Creatinine (mg/dL) | 0.9 | 0.7 - 1.3 |
| Urea (mg/dL) | 37 | 18 - 55 |
| Sodium (mmol/L) | 137 | 136 - 145 |
| Potassium (mmol/L) | 4.3 | 3.5 - 5.1 |
| D-dimer (ng/mL) | 915 | <230 |
| Fibrinogen (mg/dL) | 226 | 203 - 472 |
| Prothrombin time (sec) | 11.5 | 11.9 |
| Partial thromboplastin time (sec) | 25.6 | 27.9 |
Laboratory test results upon admission.
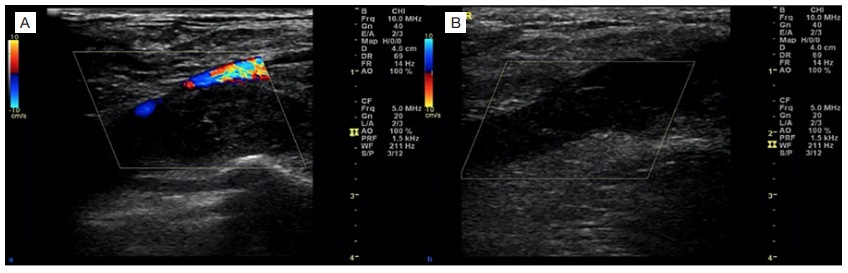
Figure 1: Doppler ultrasonography of the left upper limb ultrasound with colour Doppler of left subclavian (A) and axillary veins (B) with increased venous diameter, absent color flow and totally oclusive hypoechoic thrombus in the lumen. These findings suggest acute.
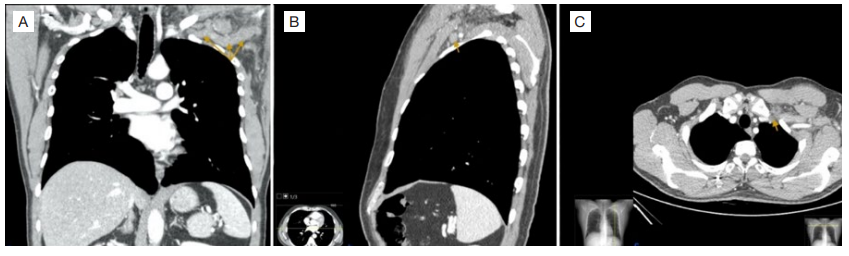
Figure 2: Thoracic contrastenhanced computed tomography. Contrast-enhanced computed tomography (A-coronal, B-sagittal, C-axial views) demonstrating dilation and decreased opacification of the left subclavian vein and left axillary vein (arrows) compatible with thrombosis.
The patient was discharged on oral anticoagulation, anal-gesia and elevation of the extremity. Further investigation (Table 2) excluded hypercoagulable or thrombophilic conditions: pro-thrombin gene mutation (G20210A), factor V gene mutation (G1691A), antiphospholipid antibodies, protein S and C and an-tithrombin III. He continued his work as a waiter, avoiding upper limb exercises. At the 1-year follow-up, the patient was asymptomatic and without sequelae, particularly no post-thrombotic syndrome.
| Parameter | Value | Reference Value |
|---|---|---|
| Homocysteine (µmol/L) | 9.98 | 5.46 - 16.20 |
| Lupus anticoagulant | Negative | Negative |
| Beta 2-glycoprotein I IgG (U/mL) | 1.20 | Negative <7 |
| Beta 2-glycoprotein I IgM (U/mL) | 1.80 | Negative <7 |
| Anticardiolipin IgG (U/mL) | <0.01 | Negative <10 |
| Anticardiolipin IgM (U/mL) | 0.20 | Negative <10 |
| Antithrombin III (%) | 94 | 83 - 128 |
| Protein C (%) | 118 | 70 - 140 |
| Protein S (%) | 97 | 74.1 - 146.1 |
Following prothrombotic investigation.
Discussion
PSS, or effort thrombosis, is an uncommon cause of pri-mary UEDVT and refers to axillary-subclavian vein thrombosis associated with strenuous and/or repetitive movements of the upper extremities.
In this report, the patient had recently started a routine of dip exercises, in which pectoral muscles (sternal, clavicular, and minor) are involved as the main synergists. In fact, certain sporting or working activities involve repetitive or vigorous hyperabduction and/or external rotation of the shoulder joint, which results in compression of the subclavian vein. This leads to endothelial microtrauma and subsequent vascular intimal hyperplasia, inflammation, fibrosis and local activation of the coagulation cascade. Furthermore, mobility of the subclavian vein may be restricted by extrinsic compression of the vein by adjacent structures at the thoracic outlet (cervical rib, congenital fiber-muscular bands, hypertrophy of the scalene, sub-clavian and pectoralis minor muscles, and abnormal insertion of the costoclavicular ligament), contributing to venous injury and consequent thrombosis. It is unclear, however, whether thrombosis results from a single insult or is the result of the cumulative effects of chronic injury.4-7
In addition to the anatomical abnormalities, described patients with thoracic outlet syndrome, caused by compression of neurovascular structures in the cervical area above the first rib, may also develop PSS (about 34% in one series).8 Apical tumours of the superior sulcus of the lung (Pancoast tumour) and thrombophilias are predisposing conditions and should be included in the etiological investigation.7
However, PSS can occur in individuals without apparent thrombotic or structural risk factors, and despite the higher incidence at a young age, this syndrome has also been described in older patients. Moreover, in some cases, thrombosis does not occur in the dominant limb and is not associated with significant upper limb activity, as noticed in this report.
Signs and symptoms extend over a wide range. Hence, the diagnostic approach should include a high index of suspicion, careful history, and clinical findings supported by appropriate imaging. Differential diagnosis include lymphedema, cellulitis, neoplastic compression of the veins, muscle injury, or superficial vein thrombosis.6,7
Venous duplex ultrasonography is the initial imaging modality, because of its availability and non-invasive features, with high sensitivity (78% to 100%) and specificity (82% to 100%) for peripheral thrombosis (jugular, distal subclavian, axillary).9 However, acoustic shadowing from the clavicle and sternum may limit visualization of the proximal veins and the ultrasonography is indeterminate.9,10In these cases, computed tomography and magnetic resonance may be useful in imaging anatomy to assess the proximal extent of DVT and possibility of compression of vascular structures.
Although less frequent when compared to lower extremity DVT, there are significant complications resulting from UEDVT. However, data on their prevalence in PSS, in particular, are unknown. Based on total UEDVT cases, PE data are heterogeneous, with rates ranging from 5% to 17% and subclinical cases can be as high as 36%.11-14Post-thrombotic syndrome is frequent (18%-44%) and is associated with significant functional disability.15,16Other complications include superior vena cava syndrome, septic thrombophlebitis, thoracic duct obstruction and brachial plexopaty.2,3,14The risk of PSS recurrence is uncertain, but rates tend to be higher in patients with concomitant neoplastic disease or vascular implantable devices. Predictably, recurrences tend to occur on the ipsilateral side. In the present case, no recurrence of PSS was observed during the follow-up period.
The mainstay of treatment is anticoagulation, aiming to obtain early venous recanalization, restore venous patency and reduce the risk of long-term complications. Anticoagulation should be continued for at least 3 months with either low molecular weight heparin, vitamin K antagonists, or direct oral anticoagulants. Thrombolysis may be considered in patients in particular situations, such as those with severe symptoms, extension of the thrombus from the subclavian to the axillary vein, symptoms <14 days, good performance status, life expectancy >1 year, and low bleeding risk. There is no consensus on thrombectomy or thrombolysis superiority over anticoagulant regimen alone.4 The use of superior vena cava filters should only be considered in those patients with contraindications for anticoagulant or who develop pulmonary embolism despite adequate anticoagulation.17 If significant venous compression is present, surgical decompression of the subclavian vein may be necessary and include resection of the first rib or clavicle.4 Finally, symptomatic control and rehabilitation are also an important part of patient’s management.
Prognosis varies based on the risk factors for thrombosis and the degree of vein occlusion. If treated properly, regardless of the conservative or surgical approach, the prognosis is generally good.
Conclusion
PSS is a rare condition in the spectrum of UEDVT invol-ving the axillary-subclavian vein. Although more frequent in young and healthy patients, PSS should be considered in patients of any age and with a history of repetitive upper limb movements. The diagnosis is based on the correlation of individual history, clinical findings and appropriate imaging modalities, such as venous duplex ultrasound and contrast computed tomography. In most patients, anticoagulation for 3 to 6 months is reasonable, but surgical decompression may be required. Despite the good overall prognosis, serious complications can occur, such as pulmonary embolism and post-thrombotic syndrome. Thus, prompt recognition and management are crucial to avoid long-term sequelae.
Declaração de Contribuição / Contributorship Statement:
Carla Pereira Fontes - Elaboração do manuscrito, Revisão critíca, Aprovação final.
A. M. Teixeira, M. Manuel, S. Fonseca - Revisão critica, Aprovação final.















